Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please solve for extent. all values are correct. Ammonia is oxidized to form nitric oxide in the following reaction: Stoichiometry Correct. Calculate the ratio of

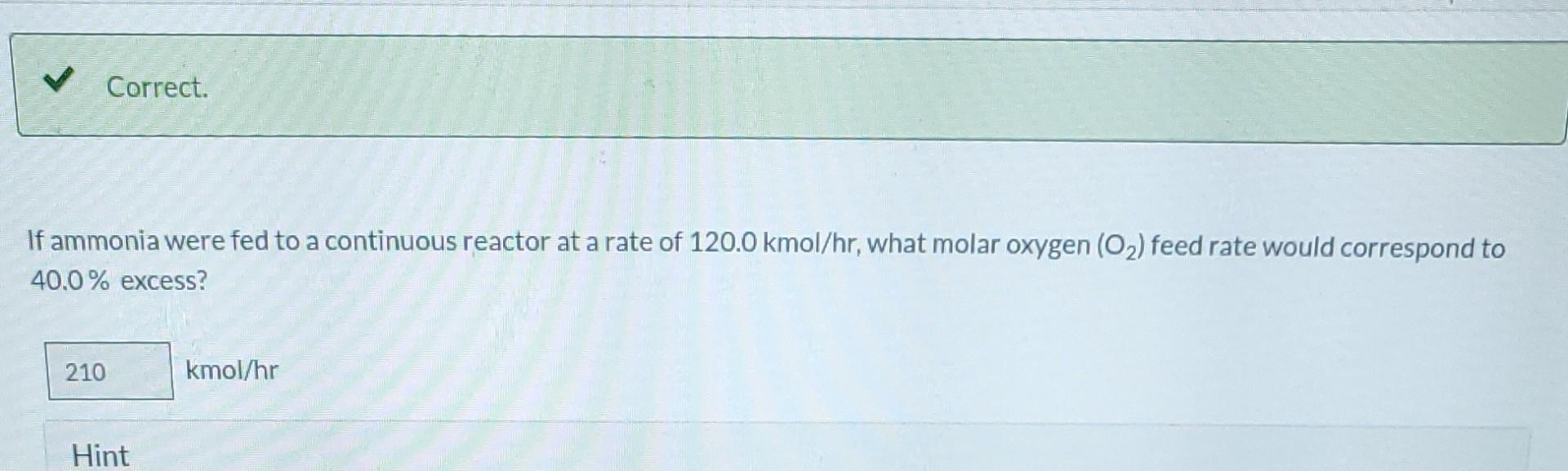

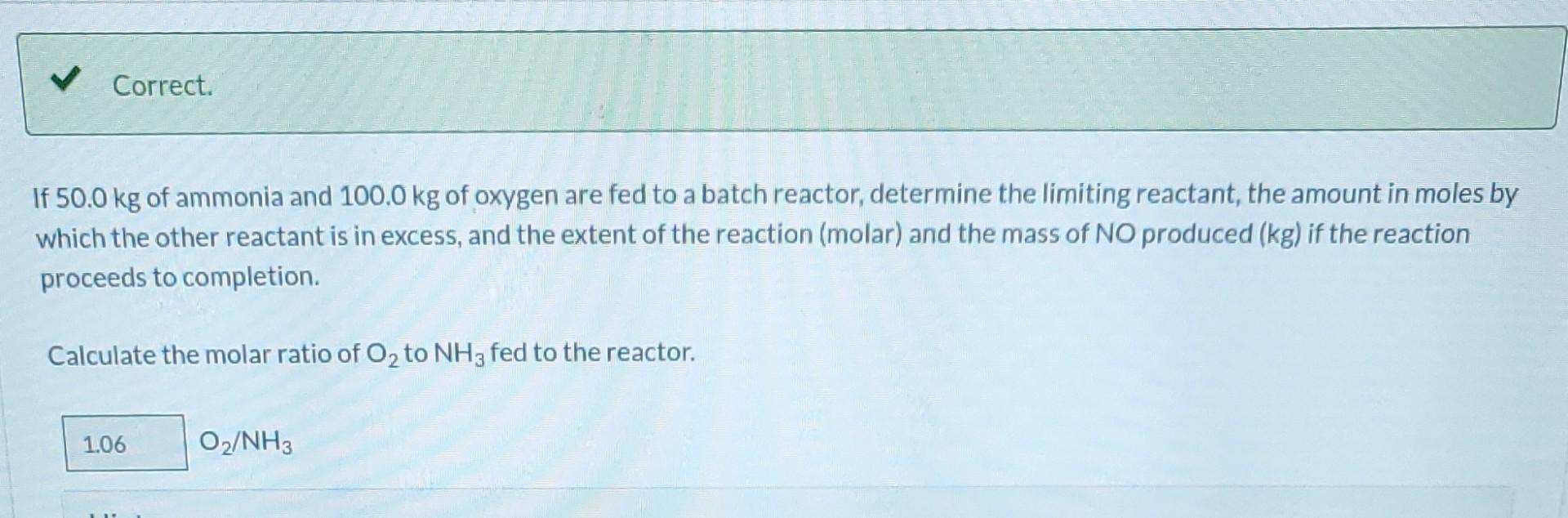
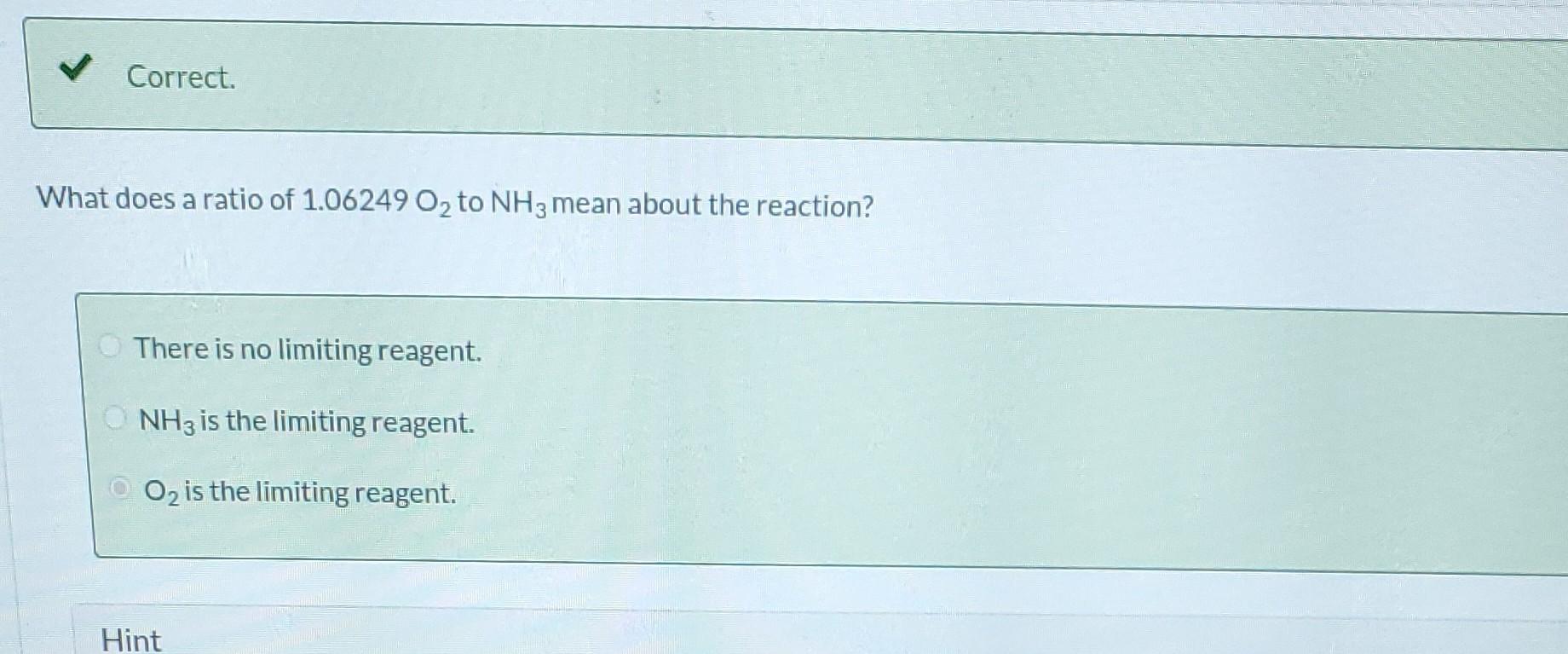
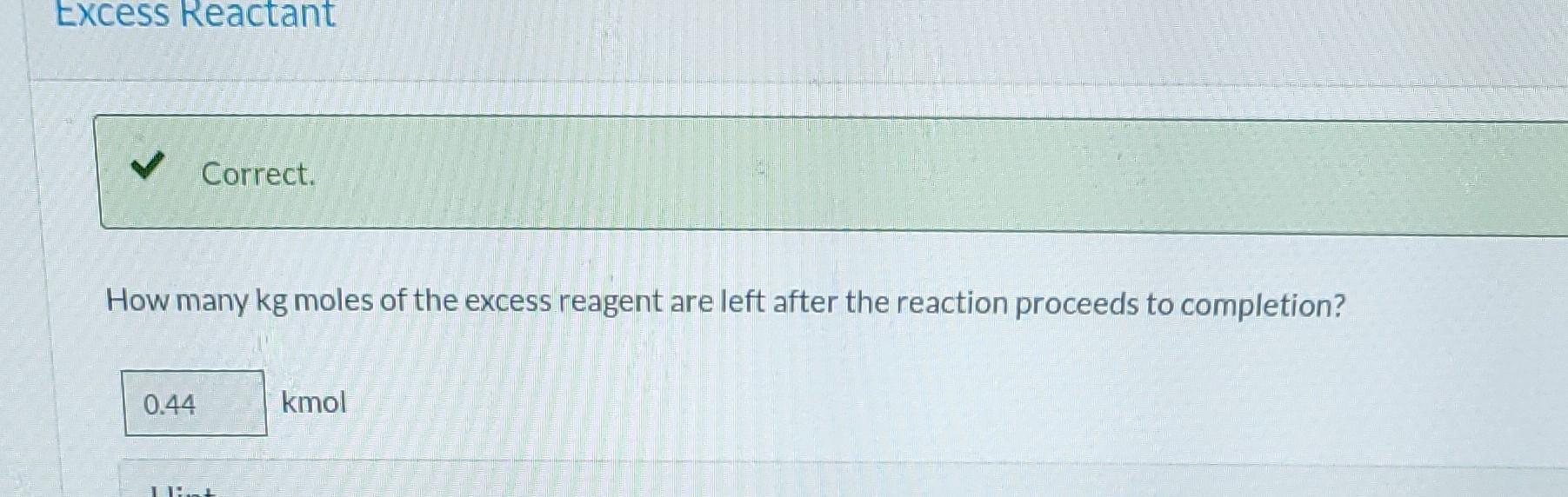
Please solve for extent. all values are correct.
Ammonia is oxidized to form nitric oxide in the following reaction: Stoichiometry Correct. Calculate the ratio of moles of O consumed to moles of NO formed. 1.25 moles O/moles NO. 4NH3 +50 NO + 6HO Correct. If ammonia were fed to a continuous reactor at a rate of 120.0 kmol/hr, what molar oxygen (O) feed rate would correspond to 40.0% excess? 210 kmol/hr Hint Correct. If 50.0 kg of ammonia and 100.0 kg of oxygen are fed to a batch reactor, determine the limiting reactant, the amount in moles by which the other reactant is in excess, and the extent of the reaction (molar) and the mass of NO produced (kg) if the reaction proceeds to completion. First find the number of moles that corresponds to 50.0 kg of ammonia (NH3). 2.94 kmol NH3 Now find the number of moles that corresponds to 100.0 kg of O. 3.125 kmol O Correct. If 50.0 kg of ammonia and 100.0 kg of oxygen are fed to a batch reactor, determine the limiting reactant, the amount in moles by which the other reactant is in excess, and the extent of the reaction (molar) and the mass of NO produced (kg) if the reaction proceeds to completion. Calculate the molar ratio of O to NH3 fed to the reactor. 1.06 02/NH3 Correct. What does a ratio of 1.06249 O to NH3 mean about the reaction? There is no limiting reagent. NH3 is the limiting reagent. O2 is the limiting reagent. Hint Excess Reactant Correct. How many kg moles of the excess reagent are left after the reaction proceeds to completion? 0.44 kmol THEL Extent of Reaction Calculate the extent of the reaction. i Save for Later Attempt Mass of NO The parts of this question must be completed in order. This part will be available when you complete thStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


