Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please solve in 45 minutes, i will thumb up Liquid propane (C3H8) enters a combustion chamber at 25C at a rate of 0.07kg/min where it
please solve in 45 minutes, i will thumb up 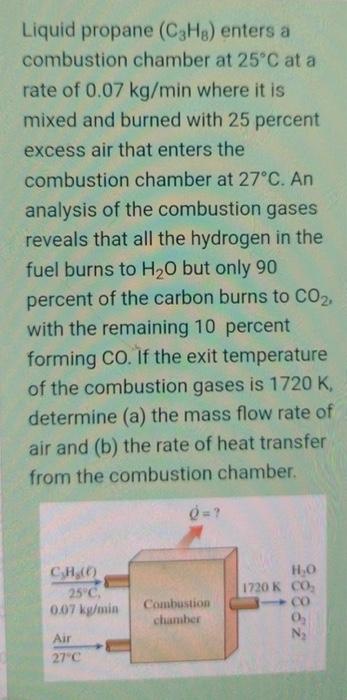
Liquid propane (C3H8) enters a combustion chamber at 25C at a rate of 0.07kg/min where it is mixed and burned with 25 percent excess air that enters the combustion chamber at 27C. An analysis of the combustion gases reveals that all the hydrogen in the fuel burns to H2O but only 90 percent of the carbon burns to CO2, with the remaining 10 percent forming CO. If the exit temperature of the combustion gases is 1720K, determine (a) the mass flow rate of air and (b) the rate of heat transfer from the combustion chamber 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


