Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please Solve it ASAP Figure Q2 shows that the chemical looping combustion (CLC) reactor at 140 kW consists of a combined unit of fuel reactor
Please Solve it ASAP
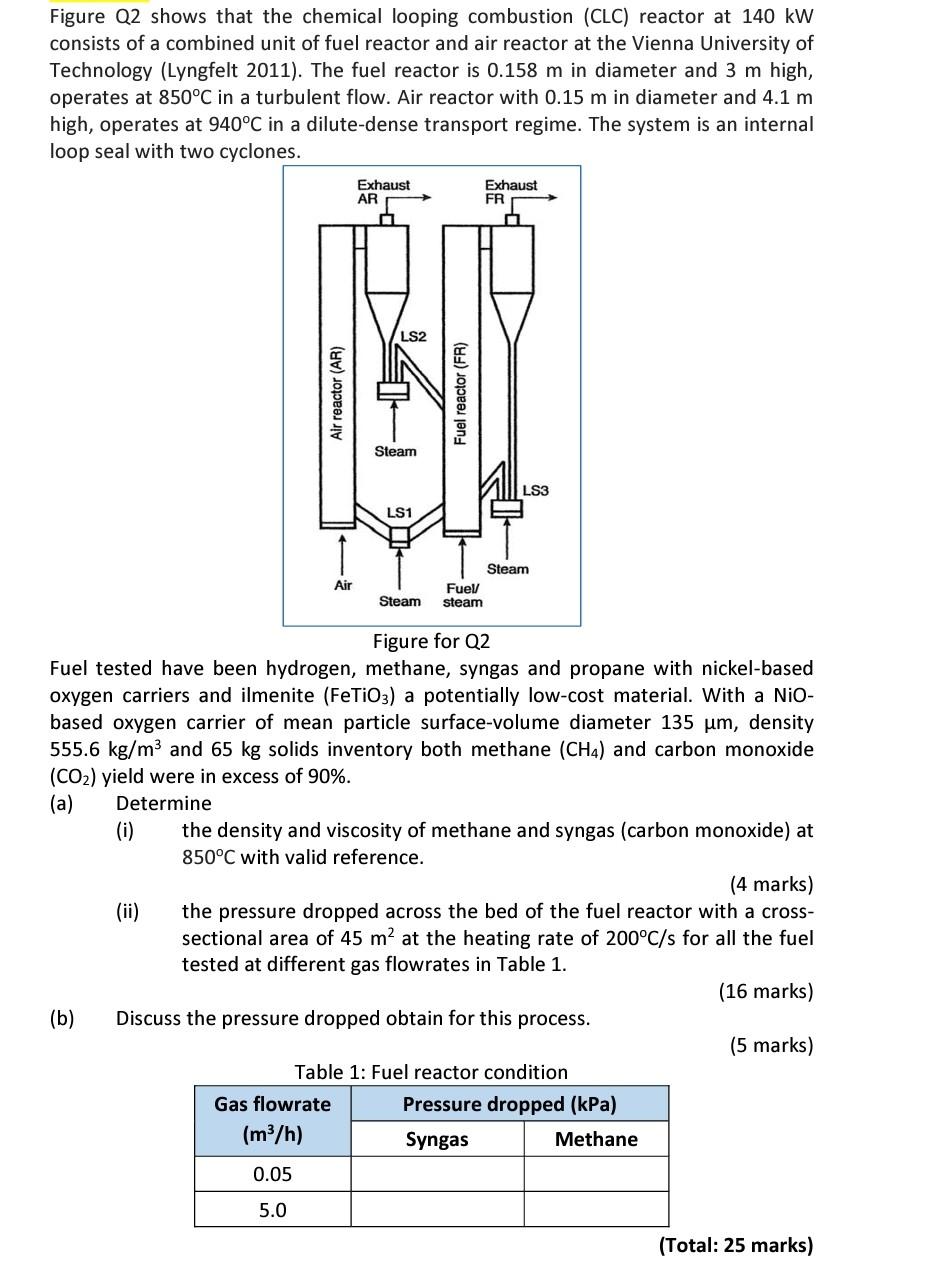
Figure Q2 shows that the chemical looping combustion (CLC) reactor at 140 kW consists of a combined unit of fuel reactor and air reactor at the Vienna University of Technology (Lyngfelt 2011). The fuel reactor is 0.158 m in diameter and 3 m high, operates at 850C in a turbulent flow. Air reactor with 0.15 m in diameter and 4.1 m high, operates at 940C in a dilute-dense transport regime. The system is an internal loop seal with two cyclones. Exhaust AR Exhaust FR LS2 Air reactor (AR) Fuel reactor (FR) Steam LS3 LS1 Air Steam Fuel steam Steam Figure for Q2 Fuel tested have been hydrogen, methane, syngas and propane with nickel-based oxygen carriers and ilmenite (FeTiO3) a potentially low-cost material. With a Nio- based oxygen carrier of mean particle surface-volume diameter 135 um, density 555.6 kg/m3 and 65 kg solids inventory both methane (CH4) and carbon monoxide (CO2) yield were in excess of 90%. (a) Determine (i) the density and viscosity of methane and syngas (carbon monoxide) at 850C with valid reference. (4 marks) the pressure dropped across the bed of the fuel reactor with a cross- sectional area of 45 m at the heating rate of 200C/s for all the fuel tested at different gas flowrates in Table 1. (16 marks) (b) Discuss the pressure dropped obtain for this process. (5 marks) Table 1: Fuel reactor condition Gas flowrate Pressure dropped (kPa) (m3/h) Syngas Methane 0.05 5.0 (Total: 25 marks)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


