Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please solve part a,b,c and d Acetylene (C2H2) is hydrogenated to form ethane (CH). The feed to the reactor contains 1.50 mol H2/mol CH2 (a)
please solve part a,b,c and d 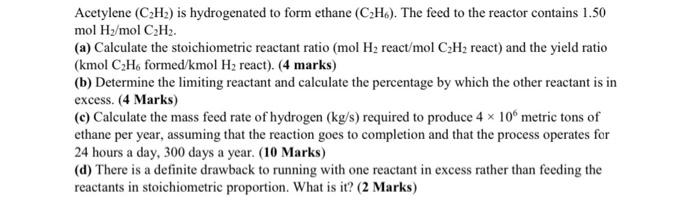
Acetylene (C2H2) is hydrogenated to form ethane (CH). The feed to the reactor contains 1.50 mol H2/mol CH2 (a) Calculate the stoichiometric reactant ratio (mol H2 react/mol CH3 react) and the yield ratio (kmol CH, formed/kmol H2 react). (4 marks) (b) Determine the limiting reactant and calculate the percentage by which the other reactant is in excess. (4 Marks) (c) Calculate the mass feed rate of hydrogen (kg/s) required to produce 4 x 10 metric tons of ethane per year, assuming that the reaction goes to completion and that the process operates for 24 hours a day, 300 days a year. (10 Marks) (d) There is a definite drawback to running with one reactant in excess rather than feeding the reactants in stoichiometric proportion. What is it? (2 Marks) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


