Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please solve part and d or just d within 2-3 hours like the solution manual - smiths thermo book 9th edition 14.20. For the ammonia
please solve part and d or just d within 2-3 hours like the solution manual - smiths thermo book 9th edition 

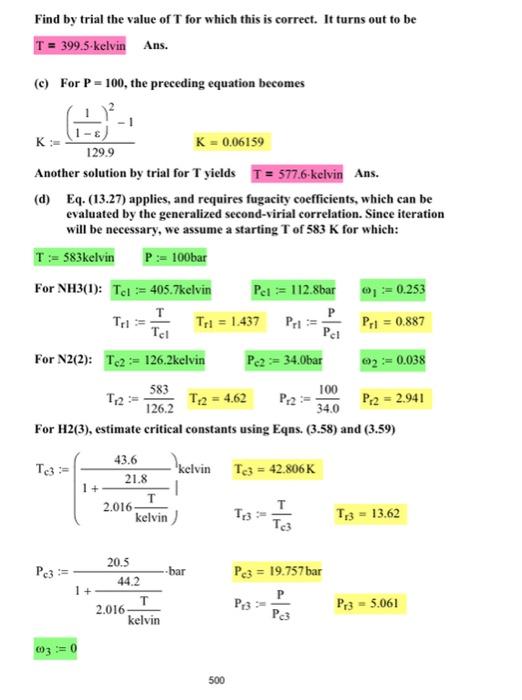
14.20. For the ammonia synthesis reaction, 21N2(g)+23H2NH3(g)v0=12123=1 the equilibrium conversion to ammonia is large at 300K, but it decreases rapidly with increasing T. However, reaction rates become appreciable only at higher temperatures. For a feed mixture of hydrogen and nitrogen in the stoichiometric proportions, R=21+28=2 (a) What is the equilibrium mole fraction of ammonia at 1 bar and 300K ? (b) At what temperature does the equilibrium mole fraction of ammonia equal 0.50 for a pressure of I bar? At what temperature does the equilibrium mole fraction of ammonia equal 0.50 for a pressure of 100 bar, assuming the equilibrium mixture is an ideal gas? At what temperature does the equilibrium mole fraction of ammonia equal 0.50 for a pressure of 100 bar, assuming the equilibrium mixture is an ideal solution of gases? yN2=21211,yH2=21231,yNH3=210+1H298=46110(234.22110.5)=46098.45JG298=16400(2317.621(8.7)=16435.75molGO1A=3.578(23)3.249(21)3.28=103B=3.02(3/2)0.422(21)0.593=05C=0D=0.186(3/2)0.083(21)0.04=. 1/2N2(g)+3/2H2(g)=NH3(g)v=1 Basis: 1/2molN2,3/2molH2 feed n0=2 This is the reaction of Pb,4.21 (a) with all stoichiometric coefficients divided by two. From the answers to Pbs. 4.21(a), 4.22(a), and 13.7(a) ALL DIVIDED BY 2 , find the following values: H298:=46110molJG298:=16450molJ A:=2.9355B:=2.0905103C:=0D:=0.3305105 (a) T:=300kelvin T0:=298.15kelvin G:=G=H298T0T(AH298G298+RIDCPH(T0,T,A,B,C,D+RTIDCPS(T0,,A,B,C,D1.627104molJK:=exp(RTG) From Pb. 13.9 for ideal gases: :=1(1+1.299KP0P)0.5=0.9664yNH3:=2yNH2=0.9349Ans. (b) For yNH3=0.5 by the preceding equation :=32 Solving the next-to-last equation for K with P=P0 gives: K:=1.299(11)21K=6.1586 Find by trial the value of T for which this is correct. It turns out to be Ans. (c) For P=100, the preceding equation becomes K:=129.9(11)21K=0.06159 Another solution by trial for T yields Ans. (d) Eq. (13.27) applies, and requires fugacity coefficients, which can be evaluated by the generalized second-virial correlation. Since iteration will be necessary, we assume a starting T of 583K for which: T:=583kelvinP:=100bar For NH3(1): Tcl:=405.7kelvin Pelcl:=112.8bar1:=0.253 Tr1:=TclTTr1=1.437Pr1:=PclPPr1=0.887 For N2(2): Tc2:=126.2kelvin Pe2:=34.0bar 2:=0.038 T12:=126.2583T12=4.62Pr2:=34.0100Pr2=2.941 For H2(3), estimate critical constants using Eqns. (3.58) and (3.59) Tc3:=1+2.016kelvinT21.8)43.6kelvinTr3:=Tc3TTc3=42.806K Pc3:=1+2.016kelvinT44.220.5barPr3=Pc3PPc3=19.757bar 3:=0 500 14.20. For the ammonia synthesis reaction, 21N2(g)+23H2NH3(g)v0=12123=1 the equilibrium conversion to ammonia is large at 300K, but it decreases rapidly with increasing T. However, reaction rates become appreciable only at higher temperatures. For a feed mixture of hydrogen and nitrogen in the stoichiometric proportions, R=21+28=2 (a) What is the equilibrium mole fraction of ammonia at 1 bar and 300K ? (b) At what temperature does the equilibrium mole fraction of ammonia equal 0.50 for a pressure of I bar? At what temperature does the equilibrium mole fraction of ammonia equal 0.50 for a pressure of 100 bar, assuming the equilibrium mixture is an ideal gas? At what temperature does the equilibrium mole fraction of ammonia equal 0.50 for a pressure of 100 bar, assuming the equilibrium mixture is an ideal solution of gases? yN2=21211,yH2=21231,yNH3=210+1H298=46110(234.22110.5)=46098.45JG298=16400(2317.621(8.7)=16435.75molGO1A=3.578(23)3.249(21)3.28=103B=3.02(3/2)0.422(21)0.593=05C=0D=0.186(3/2)0.083(21)0.04=. 1/2N2(g)+3/2H2(g)=NH3(g)v=1 Basis: 1/2molN2,3/2molH2 feed n0=2 This is the reaction of Pb,4.21 (a) with all stoichiometric coefficients divided by two. From the answers to Pbs. 4.21(a), 4.22(a), and 13.7(a) ALL DIVIDED BY 2 , find the following values: H298:=46110molJG298:=16450molJ A:=2.9355B:=2.0905103C:=0D:=0.3305105 (a) T:=300kelvin T0:=298.15kelvin G:=G=H298T0T(AH298G298+RIDCPH(T0,T,A,B,C,D+RTIDCPS(T0,,A,B,C,D1.627104molJK:=exp(RTG) From Pb. 13.9 for ideal gases: :=1(1+1.299KP0P)0.5=0.9664yNH3:=2yNH2=0.9349Ans. (b) For yNH3=0.5 by the preceding equation :=32 Solving the next-to-last equation for K with P=P0 gives: K:=1.299(11)21K=6.1586 Find by trial the value of T for which this is correct. It turns out to be Ans. (c) For P=100, the preceding equation becomes K:=129.9(11)21K=0.06159 Another solution by trial for T yields Ans. (d) Eq. (13.27) applies, and requires fugacity coefficients, which can be evaluated by the generalized second-virial correlation. Since iteration will be necessary, we assume a starting T of 583K for which: T:=583kelvinP:=100bar For NH3(1): Tcl:=405.7kelvin Pelcl:=112.8bar1:=0.253 Tr1:=TclTTr1=1.437Pr1:=PclPPr1=0.887 For N2(2): Tc2:=126.2kelvin Pe2:=34.0bar 2:=0.038 T12:=126.2583T12=4.62Pr2:=34.0100Pr2=2.941 For H2(3), estimate critical constants using Eqns. (3.58) and (3.59) Tc3:=1+2.016kelvinT21.8)43.6kelvinTr3:=Tc3TTc3=42.806K Pc3:=1+2.016kelvinT44.220.5barPr3=Pc3PPc3=19.757bar 3:=0 500 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


