Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please solve the question and Explain how you did it. 1. A simple batch distillation separates 0.6kmol of a binary feed that is 70.0mol %
Please solve the question and Explain how you did it.
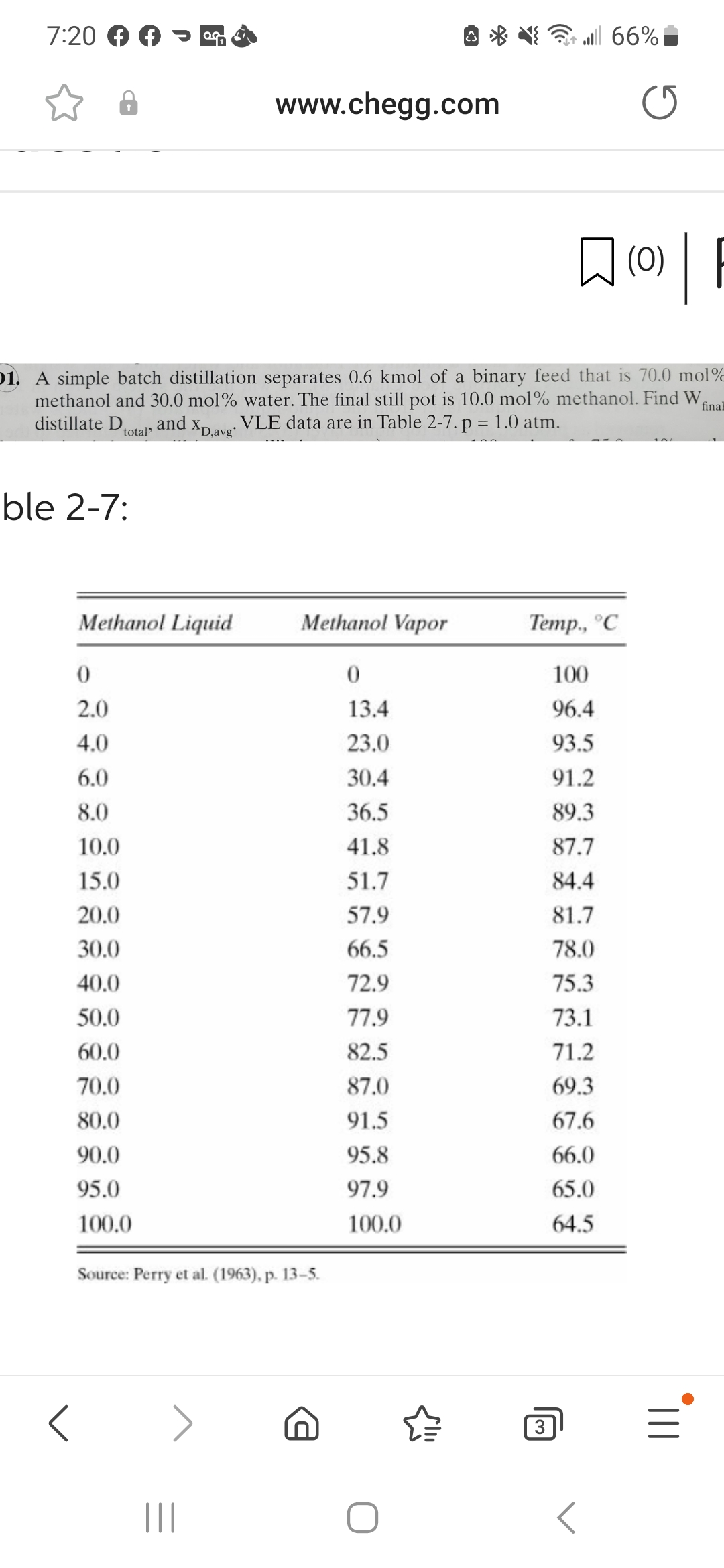 1. A simple batch distillation separates 0.6kmol of a binary feed that is 70.0mol \% methanol and 30.0mol% water. The final still pot is 10.0mol% methanol. Find Wfin distillate Dtotal, and xD,avg. VLE data are in Table 27.p=1.0atm. ble 2-7: Source: Perry el al. (1965), p. 13-2. 1. A simple batch distillation separates 0.6kmol of a binary feed that is 70.0mol \% methanol and 30.0mol% water. The final still pot is 10.0mol% methanol. Find Wfin distillate Dtotal, and xD,avg. VLE data are in Table 27.p=1.0atm. ble 2-7: Source: Perry el al. (1965), p. 13-2
1. A simple batch distillation separates 0.6kmol of a binary feed that is 70.0mol \% methanol and 30.0mol% water. The final still pot is 10.0mol% methanol. Find Wfin distillate Dtotal, and xD,avg. VLE data are in Table 27.p=1.0atm. ble 2-7: Source: Perry el al. (1965), p. 13-2. 1. A simple batch distillation separates 0.6kmol of a binary feed that is 70.0mol \% methanol and 30.0mol% water. The final still pot is 10.0mol% methanol. Find Wfin distillate Dtotal, and xD,avg. VLE data are in Table 27.p=1.0atm. ble 2-7: Source: Perry el al. (1965), p. 13-2 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


