Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please solve these problems I need the correct answer ASAP Question 1 O out of 0.34 points The gas phase irreversible reaction 2A + B
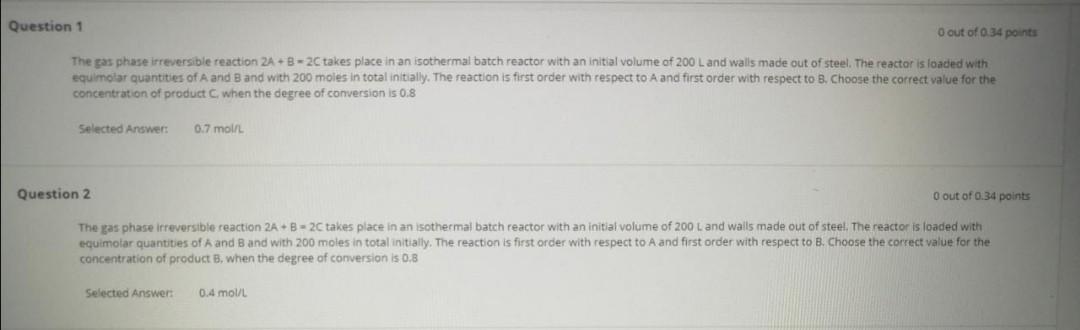
Please solve these problems I need the correct answer ASAP
Question 1 O out of 0.34 points The gas phase irreversible reaction 2A + B =2C takes place in an isothermal batch reactor with an initial volume of 200 L and walls made out of steel. The reactor is loaded with equimolar quantities of A and B and with 200 moles in total initially. The reaction is first order with respect to A and first order with respect to B. Choose the correct value for the concentration of product when the degree of conversion is 0.8 Selected Answer: 0.7 mol/l Question 2 O out of 0.34 points The gas phase irreversible reaction 2AB - 20 takes place in an isothermal batch reactor with an initial volume of 200 L and walls made out of steel. The reactor is loaded with equimotar quantities of A and B and with 200 moles in total initially. The reaction is first order with respect to A and first order with respect to B. Choose the correct value for the concentration of product B, when the degree of conversion is 0.8 Selected Answer: 0.4 mol/LStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


