Answered step by step
Verified Expert Solution
Question
1 Approved Answer
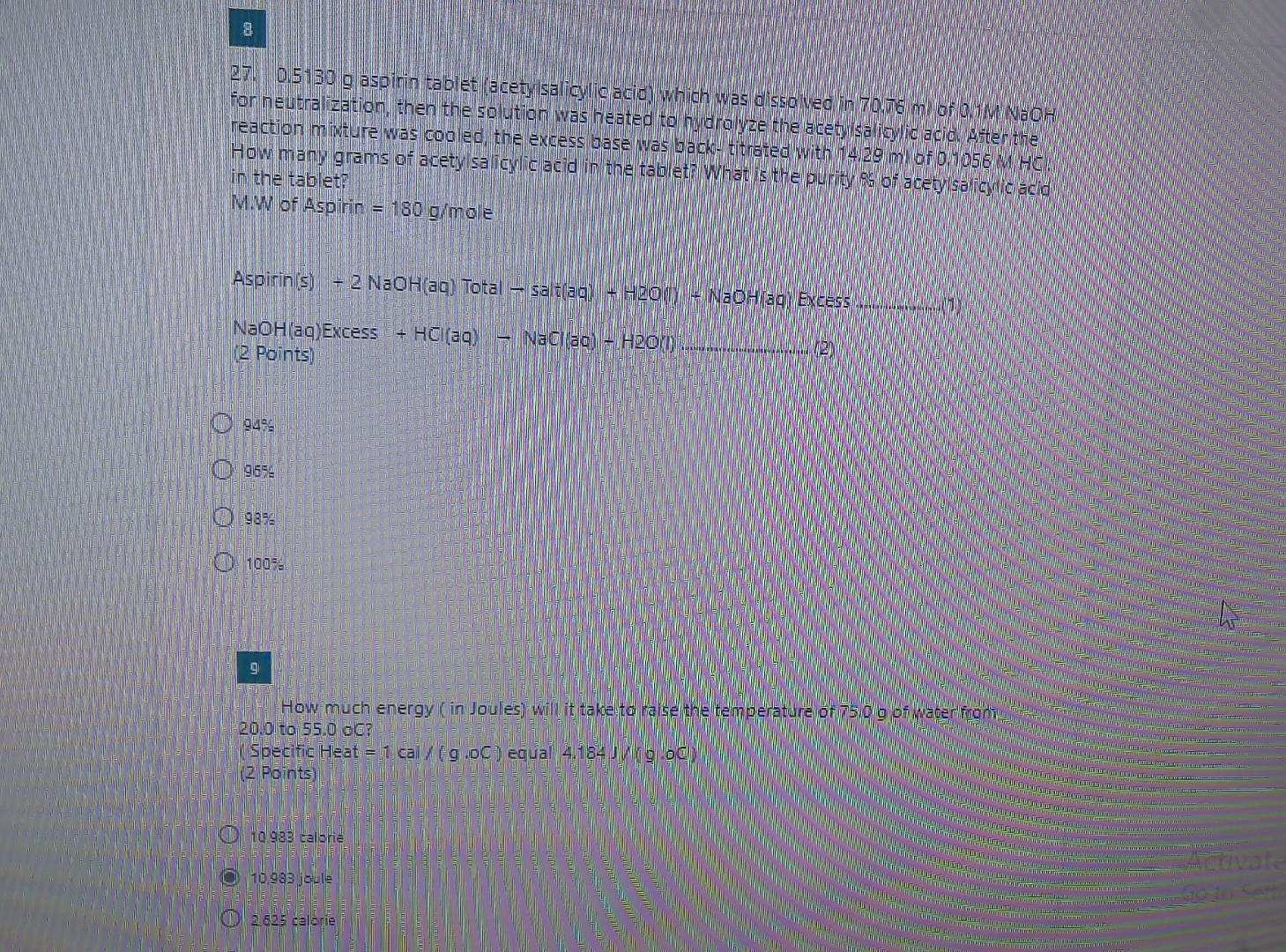
please solve this 2 questions final answer only 274 0.5130 g aspinin tablet (acetylsalicylic acid) which was dissolved in 70.76 m Of M NaOH forneubra
please solve this 2 questions final answer only
274 0.5130 g aspinin tablet (acetylsalicylic acid) which was dissolved in 70.76 m Of M NaOH forneubra ization then the so ution was heated to budra lyze the acety salicylic acid ten the reaction mixture was cooled the excess base was back titrated with 14,29 m of 0.1056 MHR How many grams of acetylsalicylic acid in the tablet What is the purity of acety sa iyo acid in the tablet MW of Aspirin = 180 g/mole Aspirin (s) 2 NaOH(aq) Total saldlag) + HbONY- NaOhlag Excess NaOH(aq)Excess 12 Points HCI() Nacaq - H2O 2 9499 0 9559 O O O O 9358 100% 9 How much energy in Joules) will it take to raise the temperature of 750g of waterfront 20.0 to 55.0 oC Specific Heat = 1 cal (9.00) equal 4.184 god (2 Points CD 10.983 calorie 0 10.983 joule 2 625 caloreStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


