please solve this. i also attach the example 6.2 and table P-4

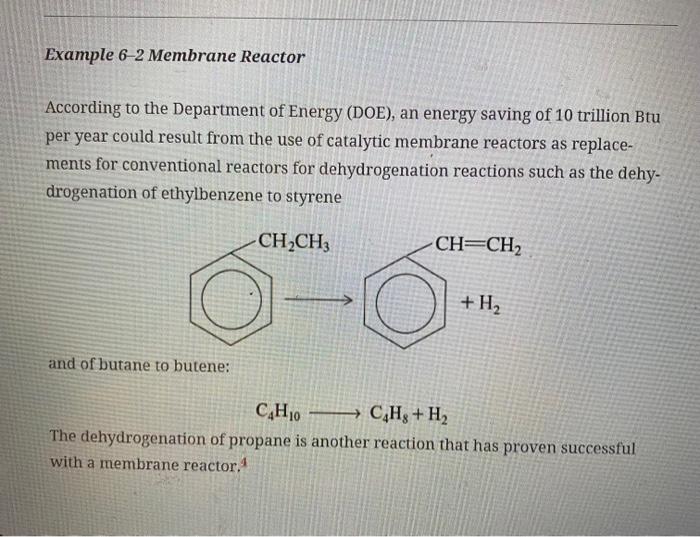
(b) Example 6-2. Download the Living Example problem 6-2 from the CRE Web site. You might find it easiest to answer most of the following questions using Wolfram. (1) Starting with all values of KC,k,CT0, and kC in the middle range (KC= 0.25,k=2.1,CT0=0.52, and kC=1.06), vary each parameter individually and describe what you find. Note and explain any maximum or minimum values of your plots down the length (i.e., volume =500dm3 ) of your reactor. Hint: Go to the extremes of the range. (2) Repeat (1) but set Kc at its maximum value and then vary k and kC and describe what you find. (3) Use Polymath to find the parameter values at which RB is a maximum and the concentrations/flow rates are at the maximum. (4) Vary ratios of parameters such as (k/kC) and (kCA0/KC)[ Note: =400 m m] and write a paragraph describing what you find. What ratio of parameters has the greatest effect on the conversion X=(FAOFAO)/FAO ? (5) Write a summary paragraph of all the trends and your results. (6) Make up a question/problem on membrane reactors with a solution in which Wolfram must be used to obtain the answer. Hint: See Preface Table P-4, page xxviii. Also comment on what types of questions would you ask when using Wolfram. Example 6-2 Membrane Reactor According to the Department of Energy (DOE), an energy saving of 10 trillion Btu per year could result from the use of catalytic membrane reactors as replacements for conventional reactors for dehydrogenation reactions such as the dehydrogenation of ethylbenzene to styrene and of butane to butene: C4H10C4H8+H2 The dehydrogenation of propane is another reaction that has proven successful with a membrane reactor. 4 One of the major goals at the undergraduate level is to bring students to the point where they can solve complex reaction problems, such as multiple reactions with heat effects, and then ask "What if ...?" questions and look for optimum operating conditions and unsafe operating conditions. The solution to one problem exemplifies this goal: the Manufacture of Styrene (Chapter 12, Problem p12-26c). This problem is particularly interesting because two reactions are endothermic and one is exothermic. (1) Ethylbenzene Styrene + Hydrogen: Endothermic (2) Ethylbenzene Benzene + Ethylene: Endothermic (3) Ethylbenzene + Hydrogen Toluene + Methane: Exothermic