Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please solve this proble in detail. the subject i am learning is reaction chemistry 2. [40] In a PFR (plug flow reactor) under steady state,
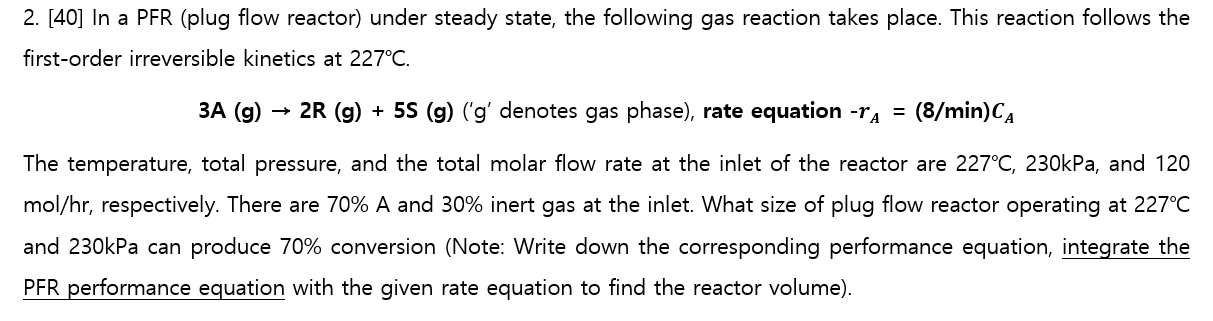
please solve this proble in detail. the subject i am learning is reaction chemistry
2. [40] In a PFR (plug flow reactor) under steady state, the following gas reaction takes place. This reaction follows the first-order irreversible kinetics at 227C. 3A(g)2R(g)+5S(g)(gdenotesgasphase),rateequationrA=(8/min)CA The temperature, total pressure, and the total molar flow rate at the inlet of the reactor are 227C,230kPa, and 120 mol/hr, respectively. There are 70%A and 30% inert gas at the inlet. What size of plug flow reactor operating at 227C and 230kPa can produce 70% conversion (Note: Write down the corresponding performance equation, integrate the PFR performance equation with the given rate equation to find the reactor volume)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


