Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please solve this questions a) Calculate AHRx, ACp, and AHRX (400 K) for the reaction A+B 2C from the information provided in the following table:
please solve this questions 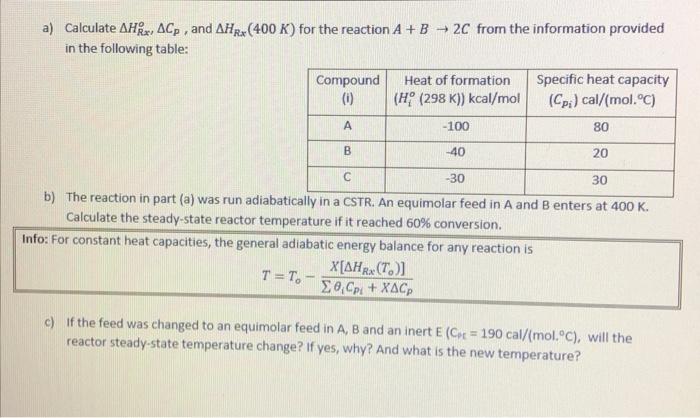
a) Calculate AHRx, ACp, and AHRX (400 K) for the reaction A+B 2C from the information provided in the following table: Compound Heat of formation Specific heat capacity (0) (H (298 K)) kcal/mol (Cpi) cal/(mol.C) -100 80 B -40 20 -30 30 b) The reaction in part (a) was run adiabatically in a CSTR. An equimolar feed in A and B enters at 400 K. Calculate the steady-state reactor temperature if it reached 60% conversion. Info: For constant heat capacities, the general adiabatic energy balance for any reaction is X[AHRx(7.)] T=T. - Cp: + XACP c) If the feed was changed to an equimolar feed in A, B and an inert E (Cor= 190 cal/(mol.c), will the reactor steady-state temperature change? If yes, why? And what is the new temperature 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


