 Please solve this with engineering equation solver (EES) code to prove the code works! Thanks!
Please solve this with engineering equation solver (EES) code to prove the code works! Thanks!
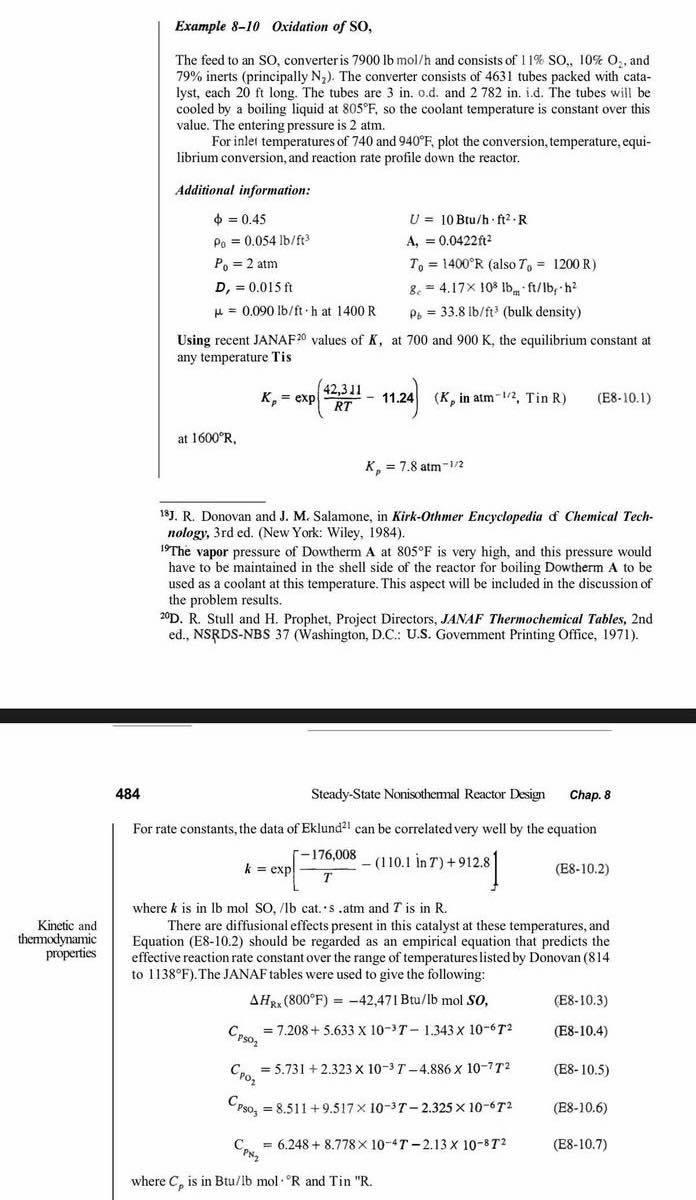
Example 8-10 Oxidation of SO, The feed to an SO, converter is 7900lbmol/h and consists of 11%SO,10%O2, and 79% inerts (principally N2 ). The converter consists of 4631 tubes packed with catalyst, each 20ft long. The tubes are 3 in. o.d. and 2782 in. i.d. The tubes will be cooled by a boiling liquid at 805F, so the coolant temperature is constant over this value. The entering pressure is 2atm. For inler temperatures of 740 and 940F, plot the conversion, temperature, equilibrium conversion, and reaction rate profile down the reactor. Additional information: =0.450=0.054lb/ft3P0=2atmD1=0.015ft=0.090lb/fthat1400RU=10Btu/hft2RA,=0.0422ft2T0=1400R(alsoT0=1200R)gc=4.17108lbmft/bbfh2b=33.8lb/ft3(bulkdensity) Using recent JANAF 20 values of K, at 700 and 900K, the equilibrium constant at any temperature Tis at 1600R, Kp=7.8atm1/2 18 J. R. Donovan and J. M. Salamone, in Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed. (New York: Wiley, 1984). 19 The vapor pressure of Dowtherm A at 805F is very high, and this pressure would have to be maintained in the shell side of the reactor for boiling Dowtherm A to be used as a coolant at this temperature. This aspect will be included in the discussion of the problem results. 20D. R. Stull and H. Prophet, Project Directors, JANAF Thermochemical Tables, 2nd ed., NSRDS-NBS 37 (Washington, D.C.: U.S. Govemment Printing Office, 1971). 484 Steady-State Nonisothemal Reactor Design Chap. 8 For rate constants, the data of Eklund 21 can be correlated very well by the equation k=exp[T176,008(110.1inT)+912.8] (E810.2) where k is in lb mol SO, /lb cat. s. atm and T is in R. There are diffusional effects present in this catalyst at these temperatures, and themodynamic Equation (E8-10.2) should be regarded as an empirical equation that predicts the properties effective reaction rate constant over the range of temperatures listed by Donovan ( 814 to 1138F ). The JANAF tables were used to give the following: HRx(800F)=42,47IBtu/lbmolSO,CPSO2=7.208+5.633103T1.343106T2CPO2=5.731+2.323103T4.886107T2CPSO3=8.511+9.517103T2.325106T2CPN2=6.248+8.778104T2.13108T2 (E8-10.3) (E8-10.4) (E810.5) (E810.6) (E810.7) where Cp is in Btu/lb mol R and Tin "R
 Please solve this with engineering equation solver (EES) code to prove the code works! Thanks!
Please solve this with engineering equation solver (EES) code to prove the code works! Thanks! 





