Question
Please solve what is requested with the data of the problem, thank you. Cumene (C6H5C3H7) is produced by the reaction of benzene and propylene in
Please solve what is requested with the data of the problem, thank you.
Cumene (C6H5C3H7) is produced by the reaction of benzene and propylene in a fixed bed catalytic reactor [r (77 F) = -39,520 Btu/lb-mol). A liquid containing 75 mol% propylene and 25 mol% n-butane is fed to the reactor, and a second liquid flow containing essentially pure benzene. Fresh and recirculated benzene, both at 77F, are mixed at a 1:3 ratio, and pass through a heat exchanger, where they are heated by reactor effluent before entering the reactor. The reactor effluent enters this exchanger at 400"F and leaves at 200"F. The pressure in the reactor is sufficient to keep the effluent in a liquid state.
After cooling in the heat exchanger, the effluent from the reactor enters a distillation column. All unreacted butane and propylene are removed at the top of the column and unreacted cumene and benzene at the bottom of the column and enter a second distillation column where they are separated. The benzene exiting the top of the second column is the recycle stream that is mixed with the fresh benzene feed. The cumene production speed is 1200lbm/h.
Shows the flowchart of the process.
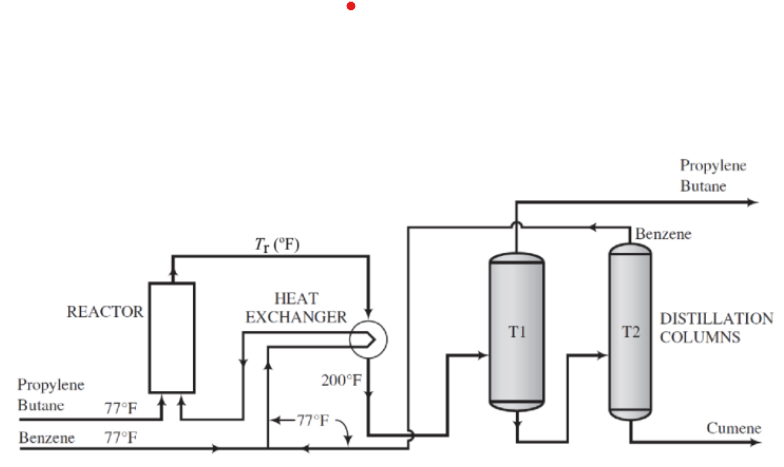
a) Determine the number of local degrees of freedom, that is, for each unit, mixing and bifurcation points.
b) Determine the number of degrees of freedom of the global process.
PLEASE.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


