Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please solve within 30 mins! A mixture of ethane, propane, n-butane, n-pentane, and n-hexane is to be separated to relatively pure components. If the split
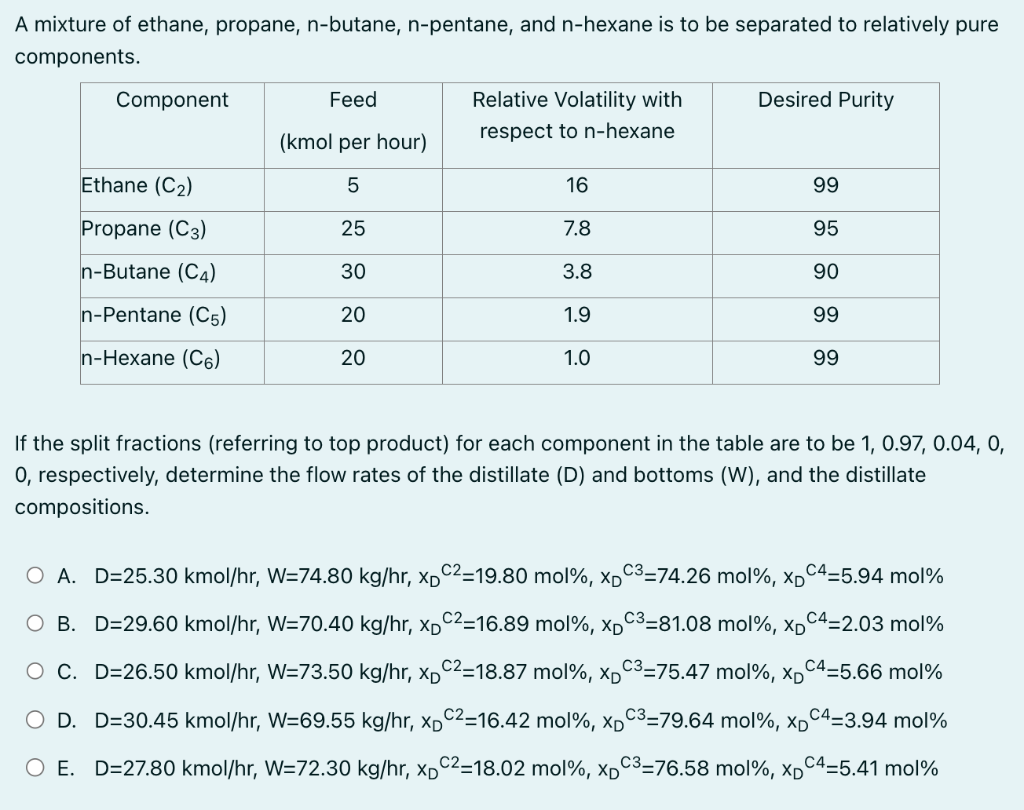
please solve within 30 mins!
A mixture of ethane, propane, n-butane, n-pentane, and n-hexane is to be separated to relatively pure components. If the split fractions (referring to top product) for each component in the table are to be 1,0.97,0.04,0, 0 , respectively, determine the flow rates of the distillate (D) and bottoms (W), and the distillate compositions. A. D=25.30kmol/hr,W=74.80kg/hr,xDC2=19.80mol%,xDC3=74.26mol%,xDC4=5.94mol% B. D=29.60kmol/hr,W=70.40kg/hr,xDC2=16.89mol%,xDC3=81.08mol%,xDC4=2.03mol%Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


