Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please someone who knows organic chemistry help me . I have a question from a lab experiment in organic chemistry. Im going to give you
Please someone who knows organic chemistry help me I have a question from a lab experiment in organic chemistry. Im going to give you information about the lab experiment. In this lab assignment, we will look in more detail at how easily alcohols, aldehydes and ketones leave
oxidize. The task must also shed light on how to detect and distinguish between carbonyl
the compounds, aldehyde and ketone. Part A: Oxidation of alcohols
Background:
During the oxidation of alcohols, different products are formed depending on whether the alcohol is primary,
secondary or tertiary. Primary alcohols are easily oxidized to aldehydes. Aldehydes allow
however, easily oxidize to carboxylic acids, so that the oxidation product from a primary alcohol is most often
the corresponding carboxylic acid. Secondary alcohols are also easily oxidized. A common one
oxidizing agent is sodium dichromate NaCrO The product will then be a ketone, which is difficult
can be oxidized further. Tertiary alcohols are generally not oxidizable. Weigh the octanol directly into a mL round bottom flask.
The oxidizing agent is prepared by mixing g KMnO and CuSO HO in
a mortar and pestle to make the mixture homogeneous.
Weigh g of the oxidizing agent into a glass weighing vessel and transfer to the round bottom flask.
The resulting reaction mixture is stirred vigorously with a spatula for one minute.
Mount a reflux condenser on the round flask and place the apparatus in a water bath of approx.
deg C Turn on the cooling water.
After hour, the reaction is complete and the reaction mixture is cooled to room temperature.
In the round bottom flask, a solid byproduct is formed and the desired oxidized product must
is desorbed from this. This is done by rinsing the contents of the flask well with hexane and then
pour the hexane into a beaker. This is done five times with mL of hexane each
time. The solid byproduct can be chopped up with a spatula while rinsing to get out
as much product as possible.
The combined hexane fractions are filtered on a sinter funnel with suction, the filtrate is dried with
MgSO in an Erlenmeyer flask and filter into a mL round bottom flask and the solvent
is evaporated on the Rota Vapor in two rounds.
Distill the residue in a Hickman column at atmospheric pressure. Increase the heat slowly
the solution begins to evaporate not above step on heating mantle Transfer it
distilled the product with a pipette into a test tube of known weight Weigh the product and
calculate the dividend.
Examine whether the product is the desired product using gas chromatography GC
For the GC analysis, mix mu l of the product with approx. ml of diethyl ether.
The GC chromatogram and the IR spectrum must be delivered together with the report and the product
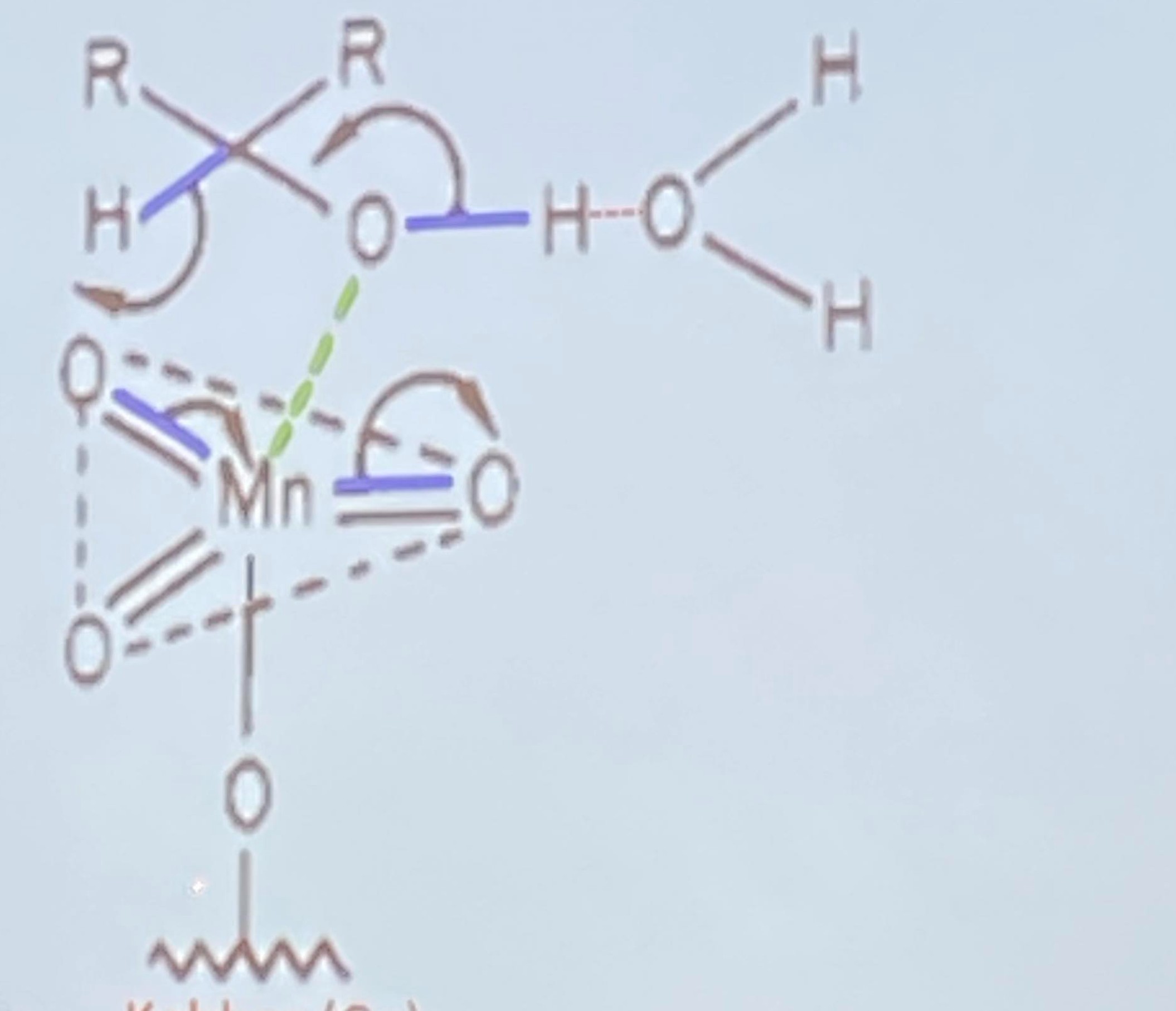
handed in on a marked sample glass and approved by the supervisor. My question is can you draw the essay for the oxidation of secondary alcohol and write what the individual parts are. If u can see the picture i inserted, its just an example of another. But it is just to show you what i mean. But remember to include with what the individual parts are called as well please.. Also please include the mechanism. And write the reaction equation. Thank you so much.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


