Answered step by step
Verified Expert Solution
Question
1 Approved Answer
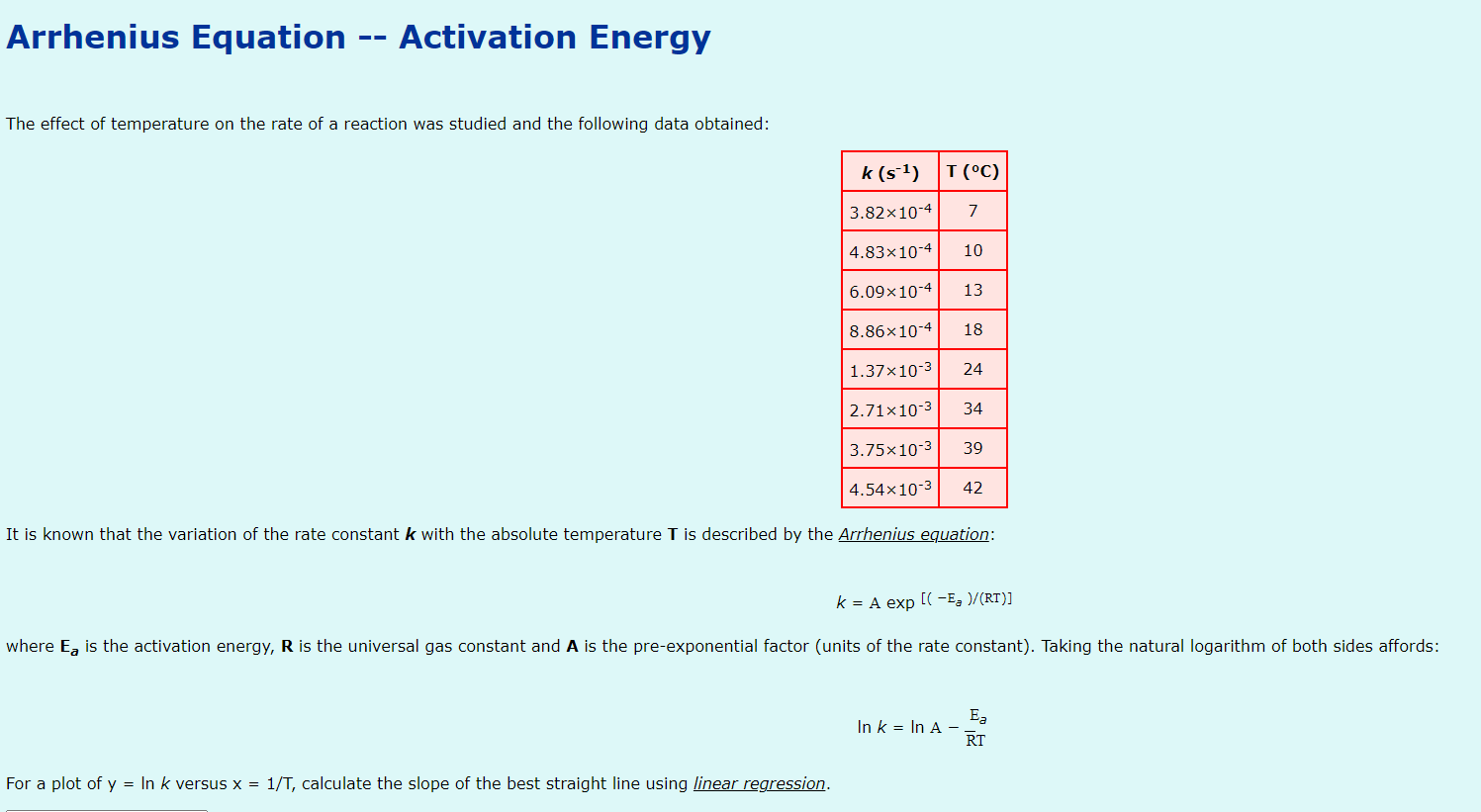
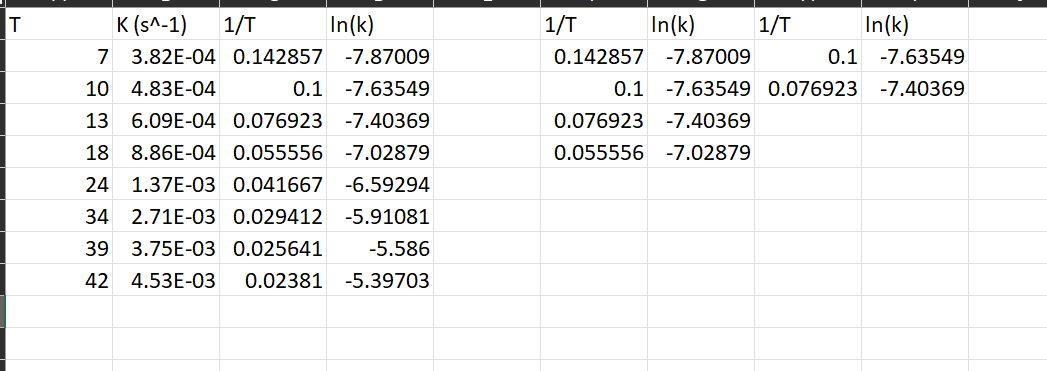
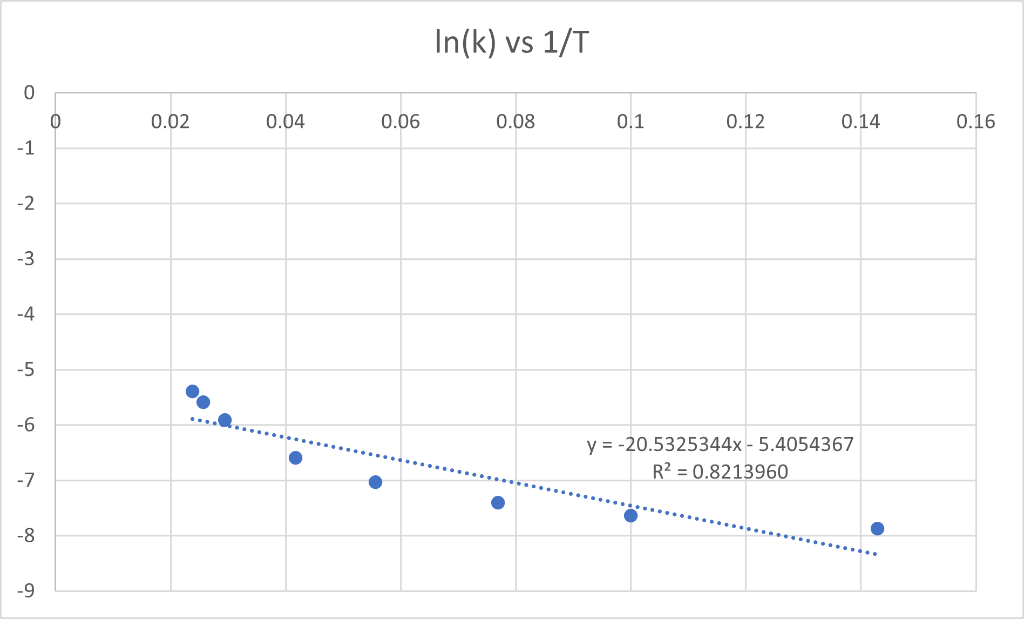
Please tell me how to find the right slope for this Arrhenius Equation/Activation Energy question, I don't understand what is meant by calculate the slope
Please tell me how to find the right slope for this Arrhenius Equation/Activation Energy question, I don't understand what is meant by "calculate the slope of the best straight line using linear regression", and googling has not been any help. I have tried removing the points that did not seem to produce a straight line from the original graph and then using excel to give the slope that way and it also was not helpful in the slightest.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


