Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please treat urgently Determine the Amount of Sodium Bicarbonate in ar Antacid Tablet Purpose: Determine the amount of sodium bicarbonate in an effervescent tablet. Materials:


please treat urgently
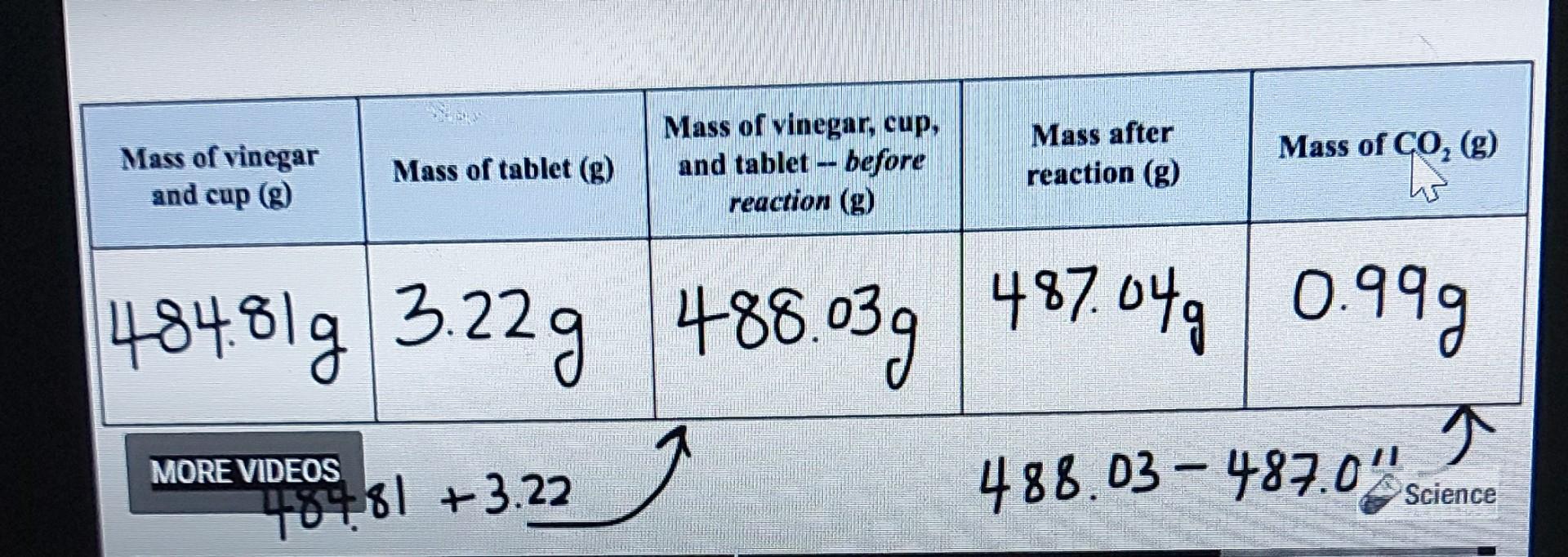
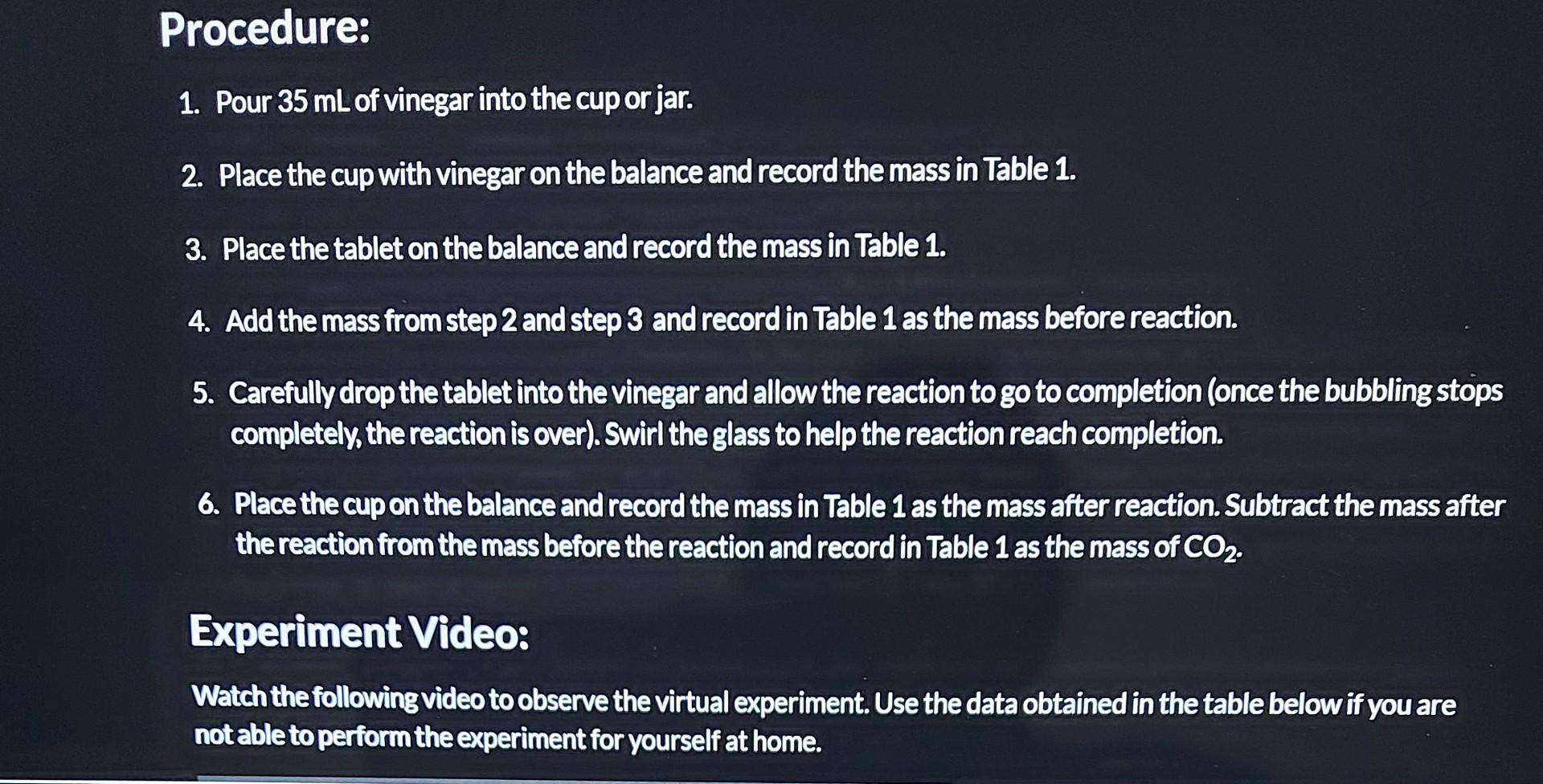
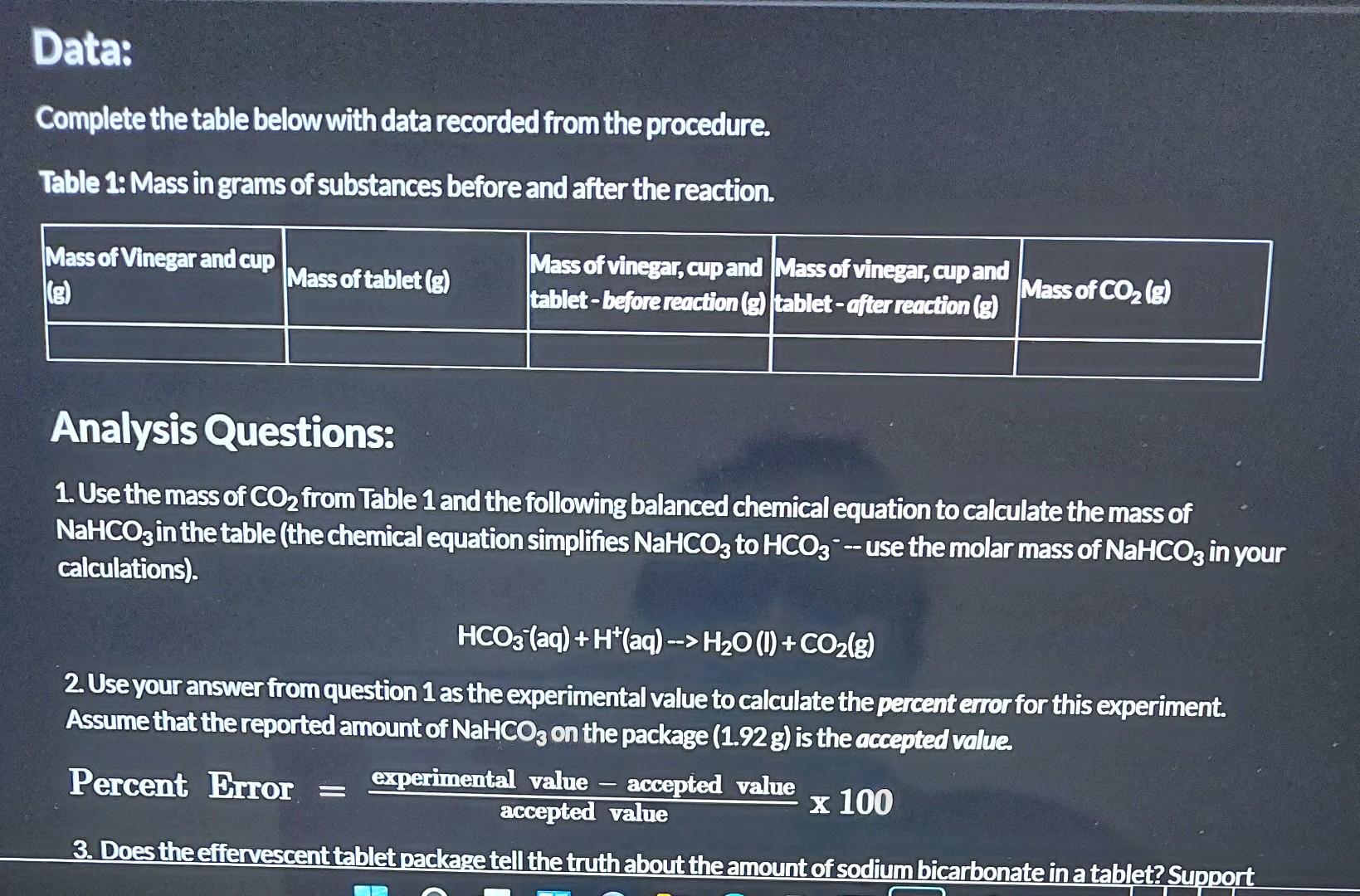
Determine the Amount of Sodium Bicarbonate in ar Antacid Tablet Purpose: Determine the amount of sodium bicarbonate in an effervescent tablet. Materials: Effervescent tablet (antacid such as Tums or Alka-Seltzer) Vinegar Large cup or jar Electronic balance or kitchen scale (preferably with 0.01 g precision) Procedure: 1. Pour 35 mL of vinegar into the cup or jar. 2. Place the cup with vinegar on the balance and record the mass in Table 1. 3. Place the tablet on the balance and record the mass in Table 1. 4. Add the mass from step 2 and step 3 and record in Table 1 as the mass before reaction. 5. Carefully drop the tablet into the vinegar and allow the reaction to go to completion (once the bubbling stops completely, the reaction is over). Swirl the glass to help the reaction reach completion. 6. Place the cup on the balance and record the mass in Table 1 as the mass after reaction. Subtract the mass after the reaction from the mass before the reaction and record in Table 1 as the mass of CO. Experiment Video: Watch the following video to observe the virtual experiment. Use the data obtained in the table below if you are not able to perform the experiment for yourself at home. Data: Complete the table below with data recorded from the procedure. Table 1: Mass in grams of substances before and after the reaction. Mass of Vinegar and cup Mass of tablet (g) Mass of vinegar, cup and Mass of vinegar, cup and tablet-before reaction (g) tablet-after reaction (g) Mass of CO (g) Analysis Questions: 1. Use the mass of CO from Table 1 and the following balanced chemical equation to calculate the mass of NaHCO3 in the table (the chemical equation simplifies NaHCO3 to HCO3 -- use the molar mass of NaHCO3 in your calculations). HCO3(aq) +H*(aq) --> HO (1) + CO(g) 2. Use your answer from question 1 as the experimental value to calculate the percent error for this experiment. Assume that the reported amount of NaHCO3 on the package (1.92 g) is the accepted value. Percent Error = experimental value accepted value accepted value x 100 3. Does the effervescent tablet package tell the truth about the amount of sodium bicarbonate in a tablet? Support 1. Use the mass of CO from Table 1 and the following balanced chemical equation to calculate the mass of NaHCO3 in the table (the chemical equation simplifies NaHCO3 to HCO3-use the molar mass of NaHCO3 in your calculations) HCO3(aq) +H*(aq)-> HO (1) + CO(g) 2. Use your answer from question 1 as the experimental value to calculate the percent error for this experiment. Assume that the reported amount of NaHCO, on the package (1.92 g) is the accepted value. Percent Error = experimental value-accepted value x 100 accepted value 3. Does the effervescent tablet package tell the truth about the amount of sodium bicarbonate in a tablet? Support your claim with evidence from the experiment. Mass of vinegar, cup, and tablet before reaction (g) Mass after reaction (g) Mass of CO (g) -- 488.03g 487.04g 0.99g 488.03-487.0" Science Mass of vinegar and cup (g) Mass of tablet (g) 484.81 g 3.22g 488.03g MORE VIDEOS 48481 +3.22
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


