Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please type 12 and 13 answers A photon has an energy of 4.211015J. What is its frequency? (h=6.62610 Js) 6.351018s12.791048s16.351018s12.791048s11.571019s1 A Question 13 (1 point)
please type 12 and 13 answers 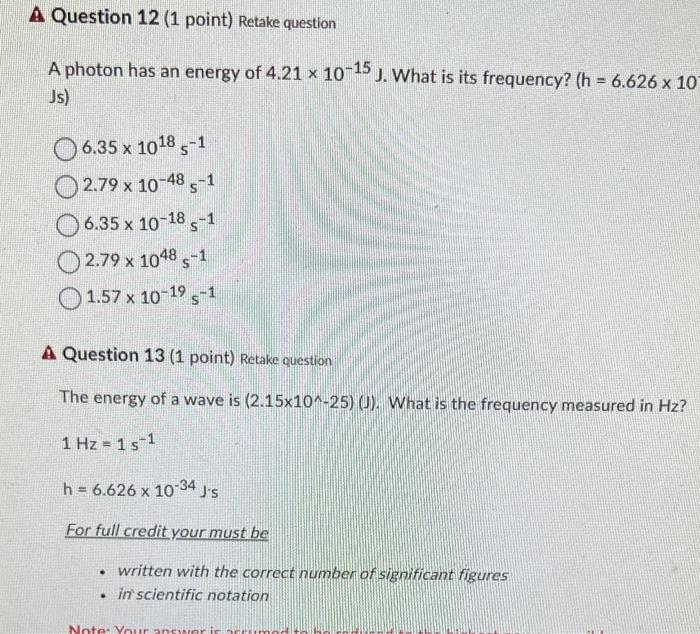
A photon has an energy of 4.211015J. What is its frequency? (h=6.62610 Js) 6.351018s12.791048s16.351018s12.791048s11.571019s1 A Question 13 (1 point) Retake question The energy of a wave is (2.151025)(J). What is the frequency measured in Hz ? 1Hz=1s1 h=6.6261034J/s For full credit your must be - written with the correct number of significant figures - in scientific notation 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


