Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please use POLYMATH to answer the conversion questions. P can be produced from A according to the first order irreversible reaction: A P , -
Please use POLYMATH to answer the conversion questions.
can be produced from A according to the first order irreversible reaction:
The reaction is carried out in a nonideal reactor, containing two immiscible liquid
phases. The compounds A P are located exclusively in the dispersed phase. The
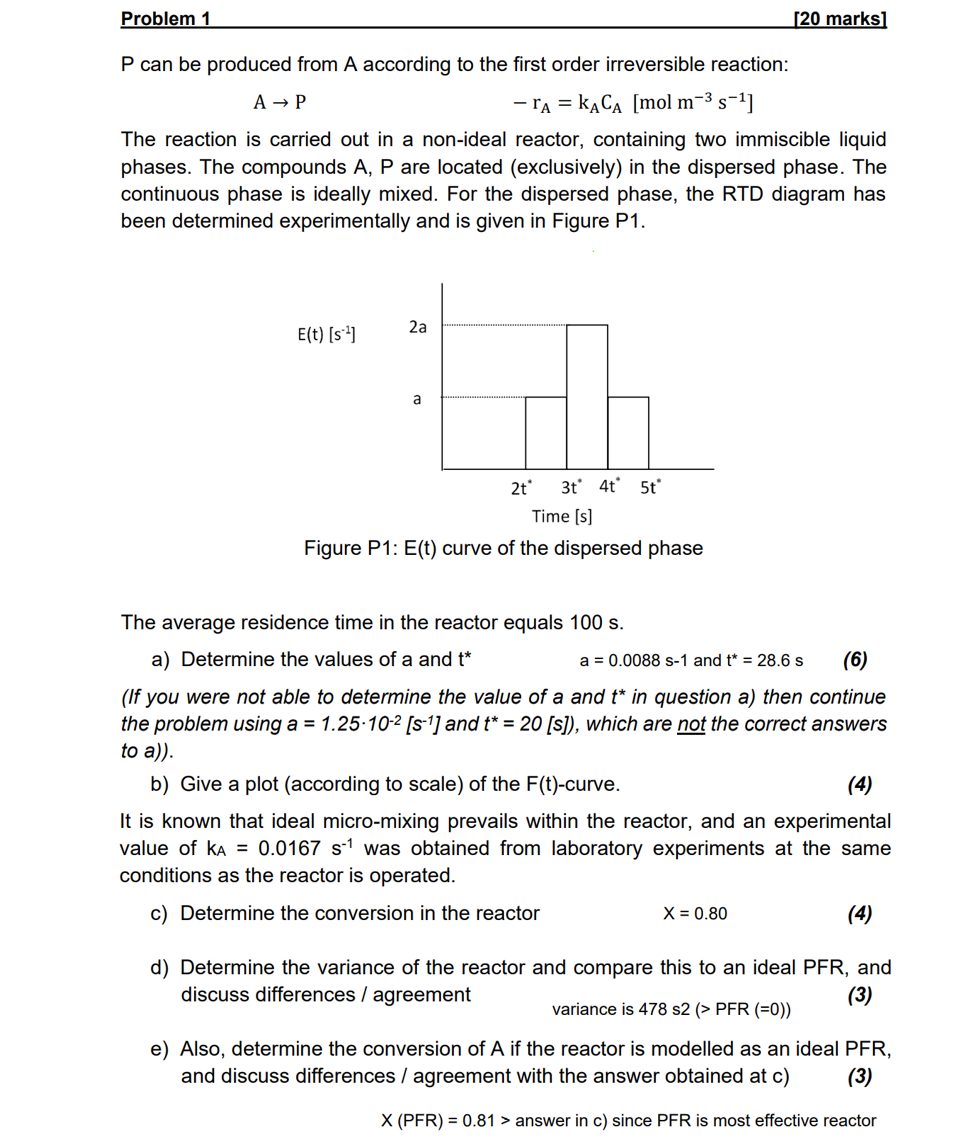
continuous phase is ideally mixed. For the dispersed phase, the RTD diagram has
been determined experimentally and is given in Figure P
Figure : curve of the dispersed phase
The average residence time in the reactor equals
a Determine the values of a and
and
If you were not able to determine the value of a and in question a then continue
the problem using and which are not the correct answers
to a
b Give a plot according to scale of the curve.
It is known that ideal micromixing prevails within the reactor, and an experimental
value of was obtained from laboratory experiments at the same
conditions as the reactor is operated.
c Determine the conversion in the reactor
d Determine the variance of the reactor and compare this to an ideal PFR and
discuss differences agreement
variance
e Also, determine the conversion of if the reactor is modelled as an ideal PFR
and discuss differences agreement with the answer obtained at c
answer
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


