Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please use the hollowing data to answer the question provided and please plot graphs. i will leave you a great rating! Using the slope value
please use the hollowing data to answer the question provided and please plot graphs. i will leave you a great rating! 
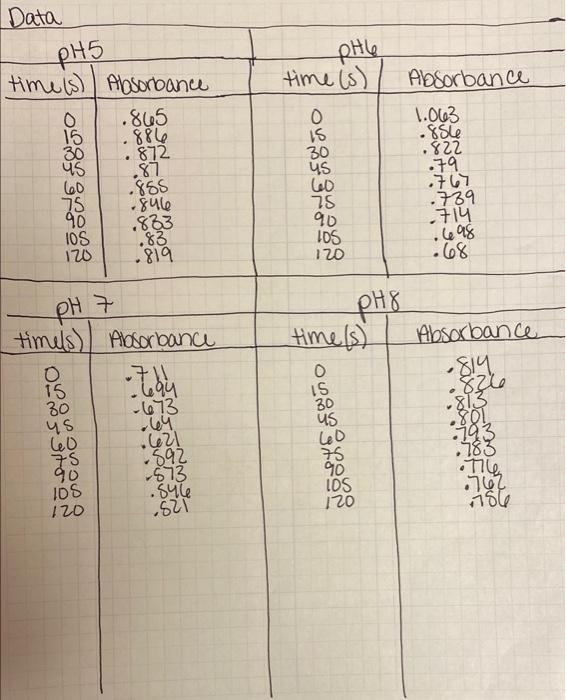
Using the slope value for the initial lincar range of a spectrunt, calculate the change in substrate concentration over time (i.e. A[fumarate]/min is the rate of the enzyme-catalyzed reaction) at each PH using the Beer's-Lambert law. Use the extinction coefficient of fumarate at 260 nm (260 900 M-lcm) and report your final answers in pimol fumarate/min (convert from AA/min). If you are unsure how to do this calculation, consult Appendix II. Spectroscopy. Plot the log of the rate of the reaction versus pH (pH is a log scale). Determine the optimal pH range for the enzyme. phle time is) Absorbance Data pH5 time is) Absorbance ..805 15 .886 .872 600 .855 75 .8416 90 .833 10S .83 120 68 1.063 .856 .822 .87 0 15 30 us 60 75 90 105 120 .79 .767 .789 .714 .698 .68 .819 pH 7 PH8 time(s) Absorbance timels) Absorbance . 15 Zbu 30 T3 us +64 .621 892 -573 .sule .621 O .814 826 .813 801 -793 .183 .762 156 75 90 IOS 120 774 IDS 120 Using the slope value for the initial lincar range of a spectrunt, calculate the change in substrate concentration over time (i.e. A[fumarate]/min is the rate of the enzyme-catalyzed reaction) at each PH using the Beer's-Lambert law. Use the extinction coefficient of fumarate at 260 nm (260 900 M-lcm) and report your final answers in pimol fumarate/min (convert from AA/min). If you are unsure how to do this calculation, consult Appendix II. Spectroscopy. Plot the log of the rate of the reaction versus pH (pH is a log scale). Determine the optimal pH range for the enzyme. phle time is) Absorbance Data pH5 time is) Absorbance ..805 15 .886 .872 600 .855 75 .8416 90 .833 10S .83 120 68 1.063 .856 .822 .87 0 15 30 us 60 75 90 105 120 .79 .767 .789 .714 .698 .68 .819 pH 7 PH8 time(s) Absorbance timels) Absorbance . 15 Zbu 30 T3 us +64 .621 892 -573 .sule .621 O .814 826 .813 801 -793 .183 .762 156 75 90 IOS 120 774 IDS 120 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


