Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please use the visible, highlighted numbers for calculations. See the other slides for questions. thanks A continuous, steady-state distillation column with a total condenser and
Please use the visible, highlighted numbers for calculations. See the other slides for questions. thanks 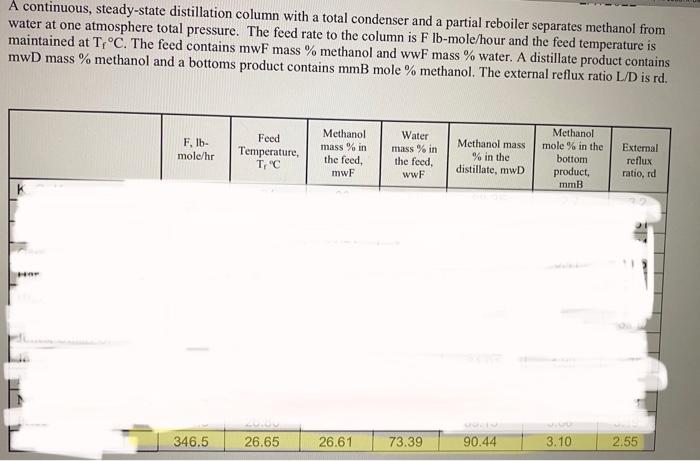
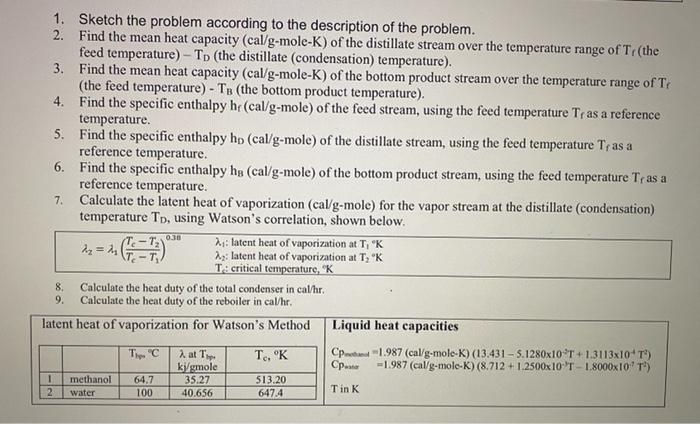
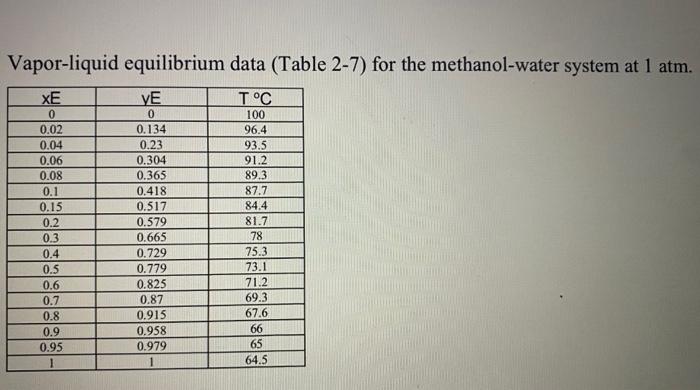
A continuous, steady-state distillation column with a total condenser and a partial reboiler separates methanol from water at one atmosphere total pressure. The feed rate to the column is Fib-mole/hour and the feed temperature is maintained at T, C. The feed contains mwF mass % methanol and wwf mass % water. A distillate product contains mwD mass % methanol and a bottoms product contains mmB mole % methanol. The external reflux ratio L/D is rd. F.lb- mole/hr Feed Temperature, T, C Methanol mass % in the feed, mwF Water mass % in the feed, wwF Methanol mass % in the distillate, mwD Methanol mole % in the bottom product, mmB External reflux ratio, rd 346.5 26.65 26.61 73.39 90.44 3.10 2.55 1. Sketch the problem according to the description of the problem. 2. Find the mean heat capacity (cal/g-mole-K) of the distillate stream over the temperature range of Tr(the feed temperature) - To the distillate (condensation) temperature). 3. Find the mean heat capacity (cal/g-mole-K) of the bottom product stream over the temperature range of T. (the feed temperature) - TB (the bottom product temperature). 4. Find the specific enthalpy h(cal/g-mole) of the feed stream, using the feed temperature Tras a reference temperature. 5. Find the specific enthalpy hp (cal/g-mole) of the distillate stream, using the feed temperature Tras a reference temperature. 6. Find the specific enthalpy he (cal/g-mole) of the bottom product stream, using the feed temperature Tras a reference temperature. 7. Calculate the latent heat of vaporization (cal/g-mole) for the vapor stream at the distillate (condensation) temperature To, using Watson's correlation, shown below. ht: latent heat of vaporization at T, "K 2 =-62) 22: latent heat of vaporization at T, K T: critical temperature, Calculate the heat duty of the total condenser in cal/hr. 9. Calculate the heat duty of the reboiler in calhr. latent heat of vaporization for Watson's Method Liquid heat capacities T. "C at T. T. OK Cp 1.987 (cal/g-mole-K) (13.431 - 5.1280x10T +1.3113x10T) ki/gmole Cp... = 1.987 (cal/g-molo-K) (8.712 +1.2500x10 T - 1.8000x1071) 35.27 Tin K 40.656 0.0 8. 1 2 methanol water 64.7 100 S13.20 647.4 Vapor-liquid equilibrium data (Table 2-7) for the methanol-water system at 1 atm. XE VE TC 0 0.02 0.04 0.06 0.08 0.1 0.15 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 0.95 1 0 0.134 0.23 0.304 0.365 0.418 0.517 0.579 0.665 0.729 0.779 0.825 0.87 0.915 0.958 0.979 1 100 96.4 93.5 91.2 89.3 87.7 84.4 81.7 78 75.3 73.1 71.2 69.3 67.6 66 65 64.5 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


