Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please verify the answers that are already there for a-c. The remainder, I cant figure out. Can you please answer and explain this problem step
Please verify the answers that are already there for a-c. The remainder, I cant figure out. Can you please answer and explain this problem step by step please.
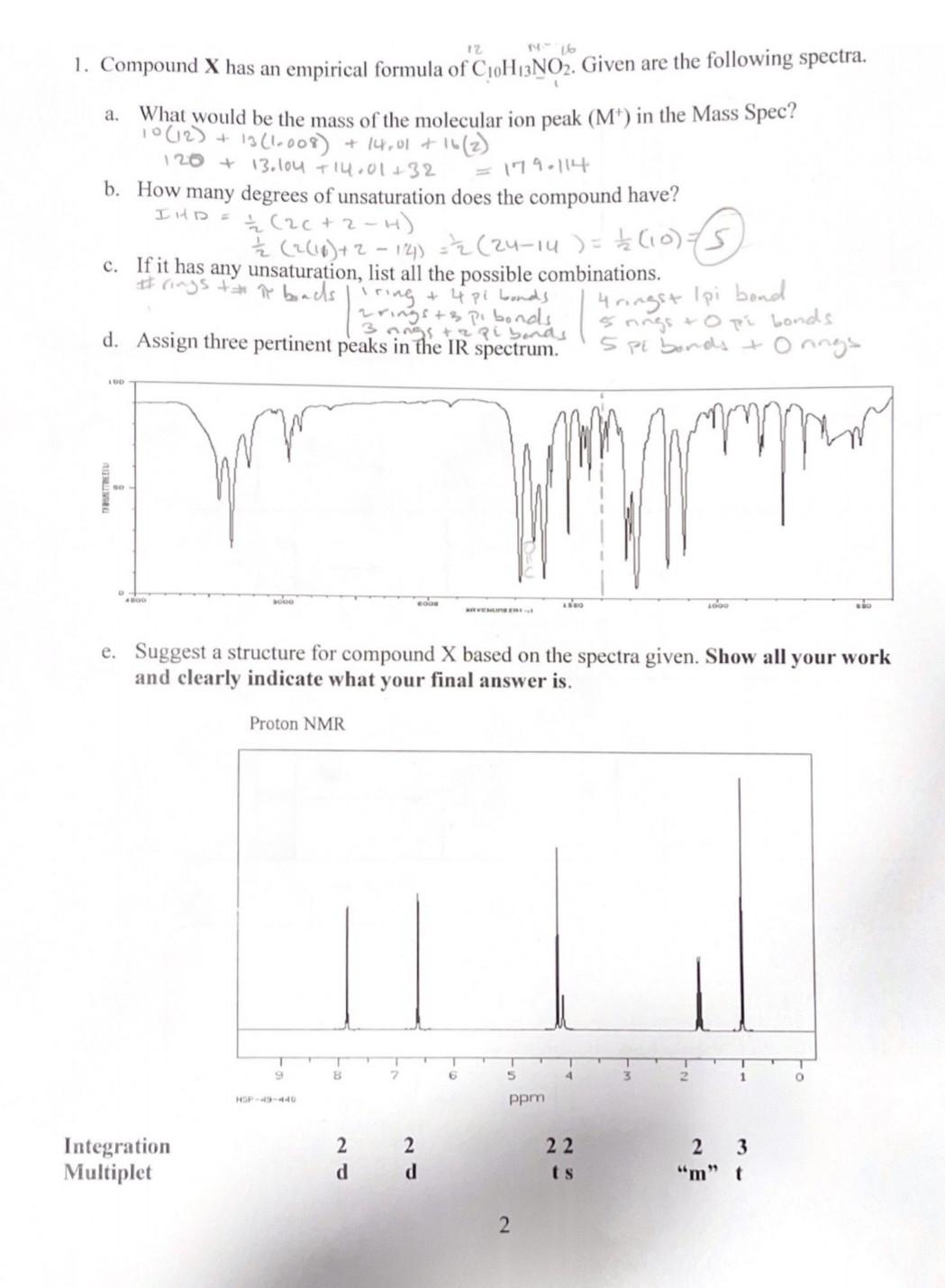
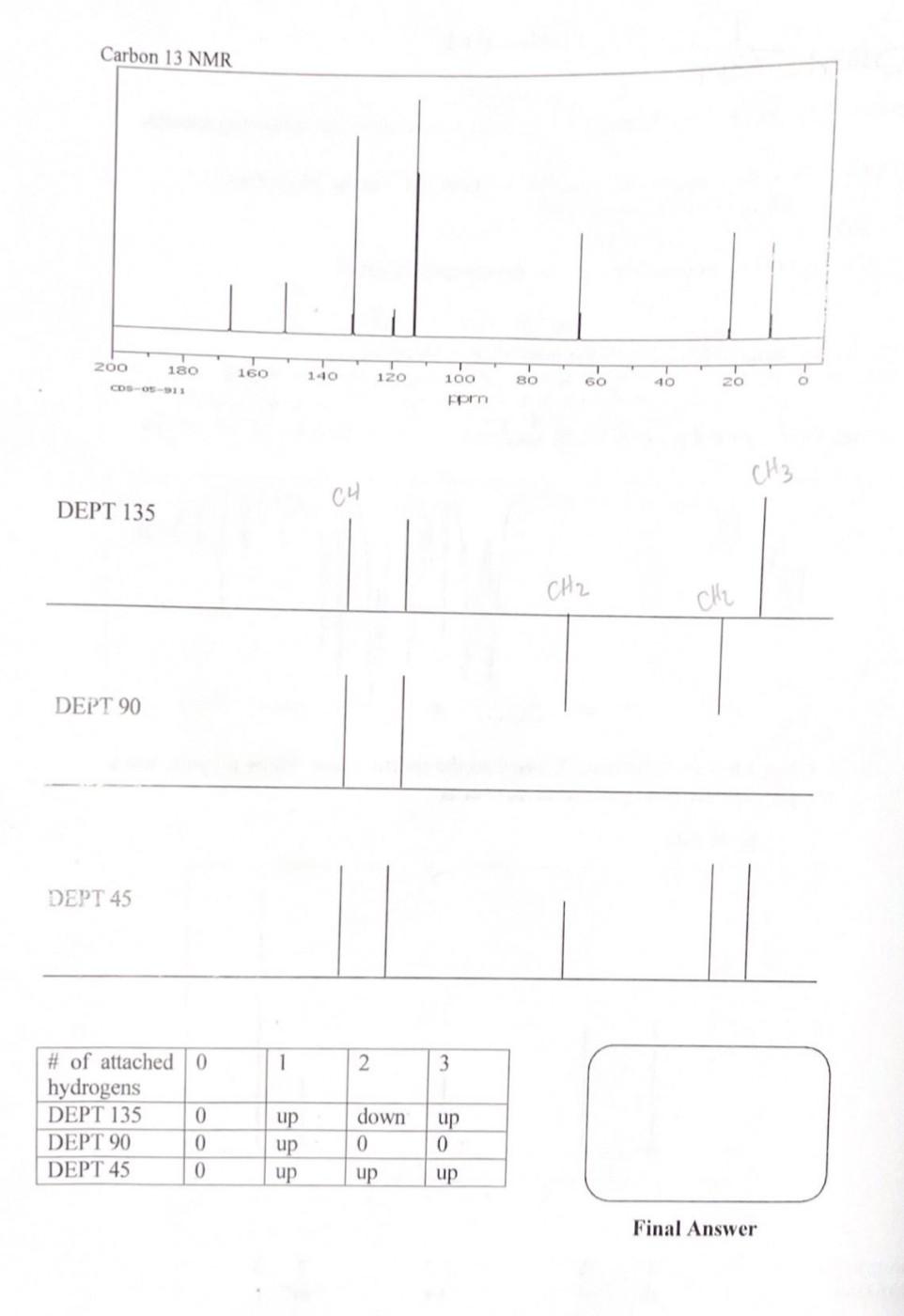
1. Compound X has an empirical formula of C10H13NO2. Given are the following spectra. a. What would be the mass of the molecular ion peak (M+)in the Mass Spec? 10(12)+13(1.008)+14.01+16(2) 120+13.104+14.01+32=179.114 b. How many degrees of unsaturation does the compound have? c. If it has any unsaturation, list all the possible combinations. d. Assign three pertinent peaks in the IR spectrum. e. Suggest a structure for compound X based on the spectra given. Show all your work and clearly indicate what your final answer is. Carbon 13 NMR DEPT 135 DEPT 90 DEPT 45 \begin{tabular}{|l|l|l|l|l|} \hline #ofattachedhydrogens & 0 & 1 & 2 & 3 \\ \hline DEPT 135 & 0 & up & down & up \\ \hline DEPT 90 & 0 & up & 0 & 0 \\ \hline DEPT 45 & 0 & up & up & up \\ \hline \end{tabular} Final
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


