Answered step by step
Verified Expert Solution
Question
1 Approved Answer
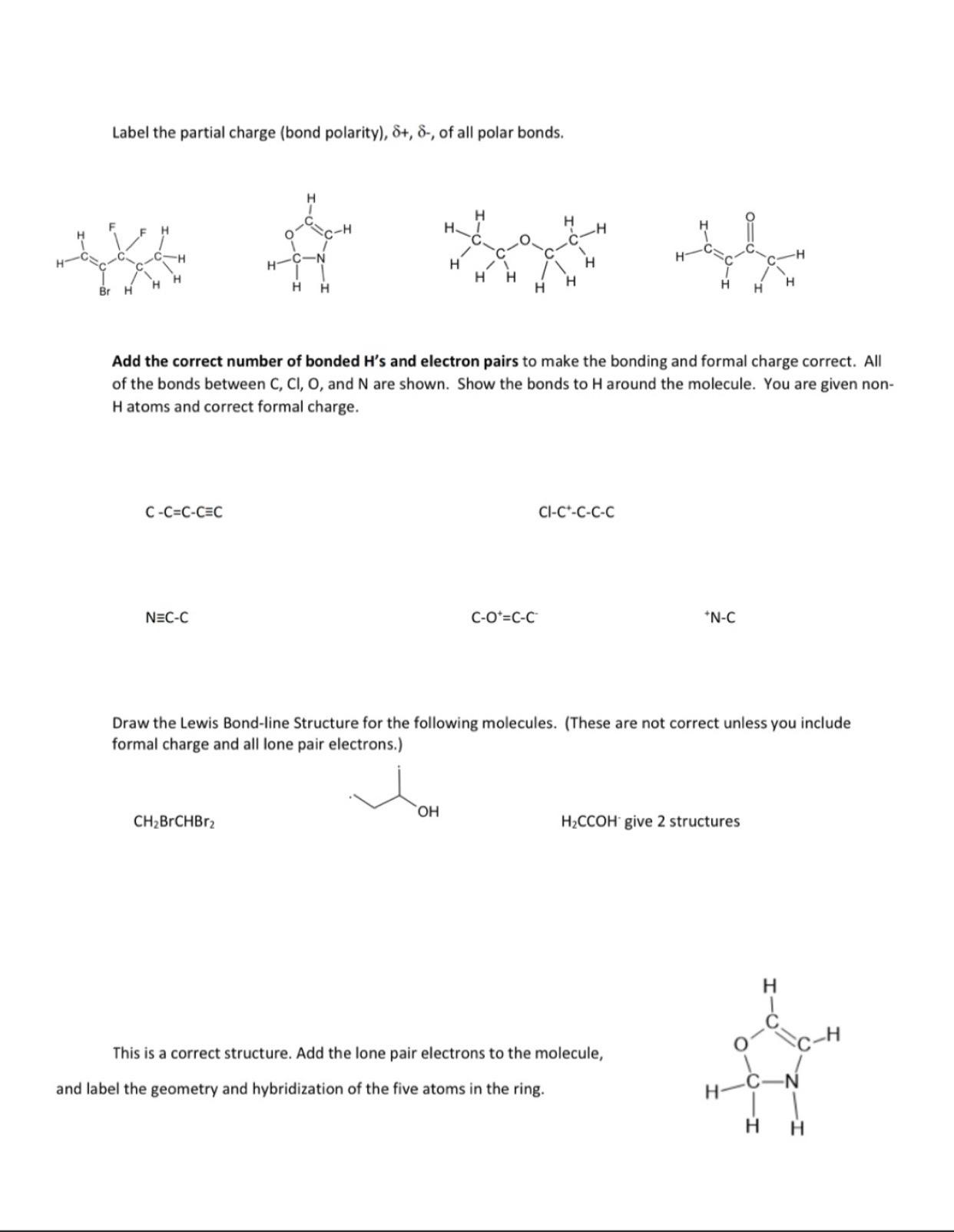
Please write rhe answer downLabel the partial charge ( bond polarity ) , + , - , of all polar bonds. Add the correct number
Please write rhe answer downLabel the partial charge bond polarity of all polar bonds.
Add the correct number of bonded s and electron pairs to make the bonding and formal charge correct. All of the bonds between and are shown. Show the bonds to around the molecule. You are given nonH atoms and correct formal charge.
Draw the Lewis Bondline Structure for the following molecules. These are not correct unless you include formal charge and all lone pair electrons.
CCOH give structures
This is a correct structure. Add the lone pair electrons to the molecule, and label the geometry and hybridization of the five atoms in the ring.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


