Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please write satements and calcutions as detailed as possible. Question: [20 marks] The vapour pressures of ethanol and water are given as: Ethanol: lnP=18.91193803.98/(T41.68) Water:
Please write satements and calcutions as detailed as possible. 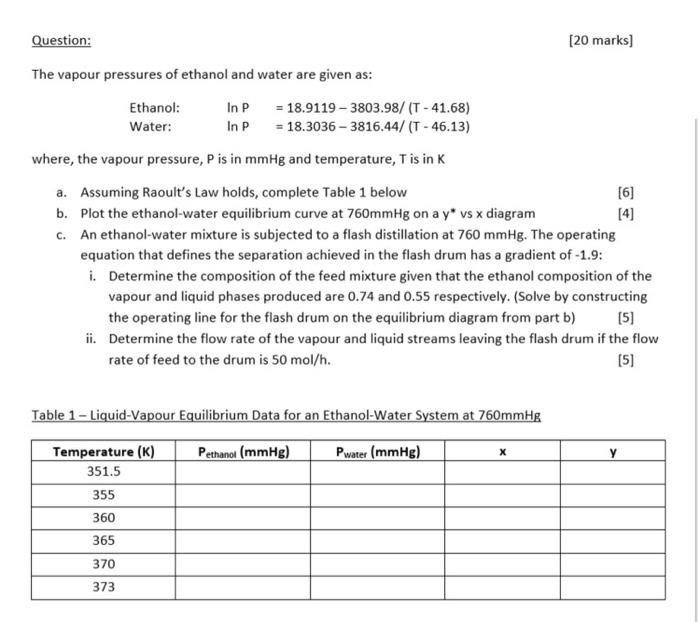
Question: [20 marks] The vapour pressures of ethanol and water are given as: Ethanol: lnP=18.91193803.98/(T41.68) Water: In P=18.30363816.44/(T46.13) where, the vapour pressure, P is in mmHg and temperature, T is in K a. Assuming Raoult's Law holds, complete Table 1 below [6] b. Plot the ethanol-water equilibrium curve at 760mmHg on a y vs x diagram [4] c. An ethanol-water mixture is subjected to a flash distillation at 760mmHg. The operating equation that defines the separation achieved in the flash drum has a gradient of -1.9 : i. Determine the composition of the feed mixture given that the ethanol composition of the vapour and liquid phases produced are 0.74 and 0.55 respectively. (Solve by constructing the operating line for the flash drum on the equilibrium diagram from part b) [5] ii. Determine the flow rate of the vapour and liquid streams leaving the flash drum if the flow rate of feed to the drum is 50mol/h. [5] Table 1 - Liquid-Vapour Equilibrium Data for an Ethanol-Water System at 760mmHg Question: [20 marks] The vapour pressures of ethanol and water are given as: Ethanol: lnP=18.91193803.98/(T41.68) Water: In P=18.30363816.44/(T46.13) where, the vapour pressure, P is in mmHg and temperature, T is in K a. Assuming Raoult's Law holds, complete Table 1 below [6] b. Plot the ethanol-water equilibrium curve at 760mmHg on a y vs x diagram [4] c. An ethanol-water mixture is subjected to a flash distillation at 760mmHg. The operating equation that defines the separation achieved in the flash drum has a gradient of -1.9 : i. Determine the composition of the feed mixture given that the ethanol composition of the vapour and liquid phases produced are 0.74 and 0.55 respectively. (Solve by constructing the operating line for the flash drum on the equilibrium diagram from part b) [5] ii. Determine the flow rate of the vapour and liquid streams leaving the flash drum if the flow rate of feed to the drum is 50mol/h. [5] Table 1 - Liquid-Vapour Equilibrium Data for an Ethanol-Water System at 760mmHg 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


