Answered step by step
Verified Expert Solution
Question
1 Approved Answer
pleasee help asap. timed chem 255 Of the 10 reactions of glycolysis, 3 are irreversible. Which one of the reactions below is one of the
pleasee help asap. timed chem 255 
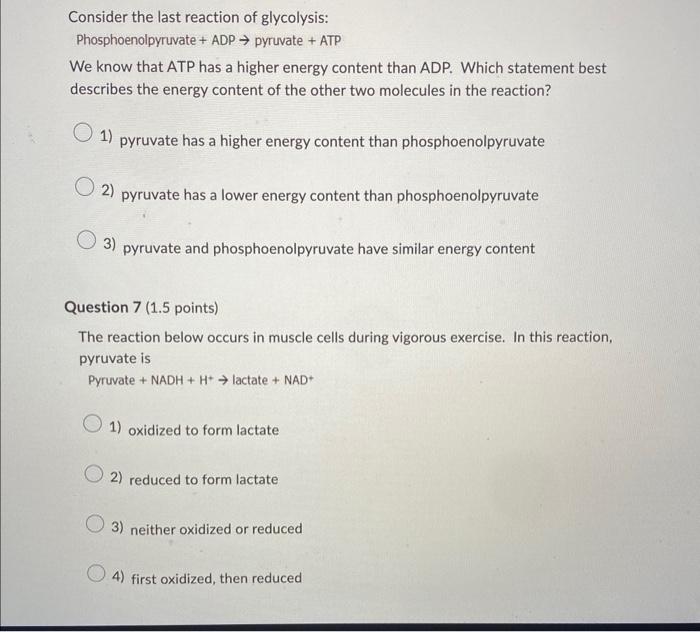
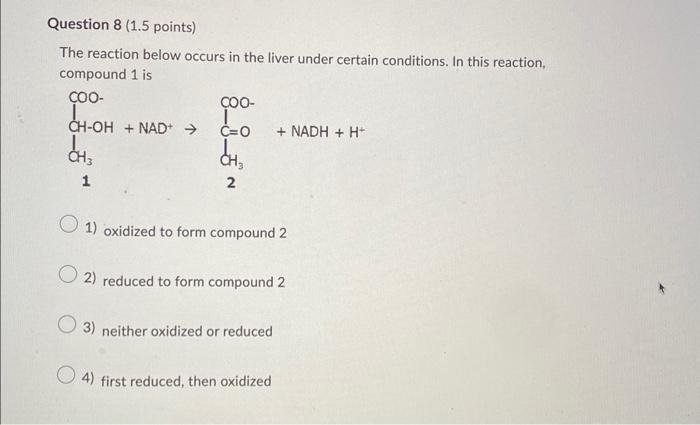
Of the 10 reactions of glycolysis, 3 are irreversible. Which one of the reactions below is one of the irreversible reactions of glycolysis? 1 Phosphoenolpyruvate + ADP pyruvate + ATP 2 Glucose-6-phosphate fructose-6-phosphate 3 3-Phosphoglycerate 2-phosphoglycerate +H2O 4 Dihydroxyacetone phosphate phosphoenolpyruvate 1) reaction 2 2) reaction 4 3) reaction 1 4) reaction 3 Consider the last reaction of glycolysis: Phosphoenolpyruvate + ADP pyruvate + ATP We know that ATP has a higher energy content than ADP. Which statement best describes the energy content of the other two molecules in the reaction? 1) pyruvate has a higher energy content than phosphoenolpyruvate 2) pyruvate has a lower energy content than phosphoenolpyruvate 3) pyruvate and phosphoenolpyruvate have similar energy content Question 7 (1.5 points) The reaction below occurs in muscle cells during vigorous exercise. In this reaction, pyruvate is Pyruvate +NADH+H+ lactate +NAD+ 1) oxidized to form lactate 2) reduced to form lactate 3) neither oxidized or reduced 4) first oxidized, then reduced The reaction below occurs in the liver under certain conditions. In this reaction, compound 1 is 1 2 1) oxidized to form compound 2 2) reduced to form compound 2 3) neither oxidized or reduced 4) first reduced, then oxidized 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


