Answered step by step
Verified Expert Solution
Question
1 Approved Answer
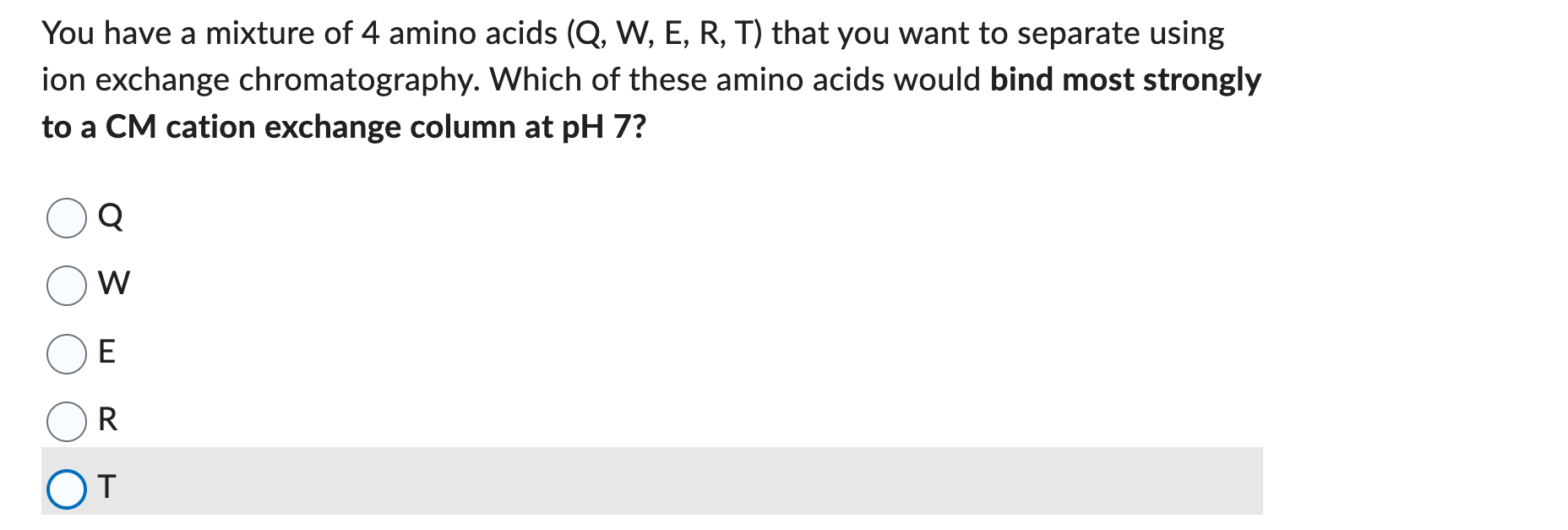
pls answer all 3 You have a mixture of 4 amino acids (Q,W,E,R,T) that you want to separate using ion exchange chromatography. Which of these

pls answer all 3
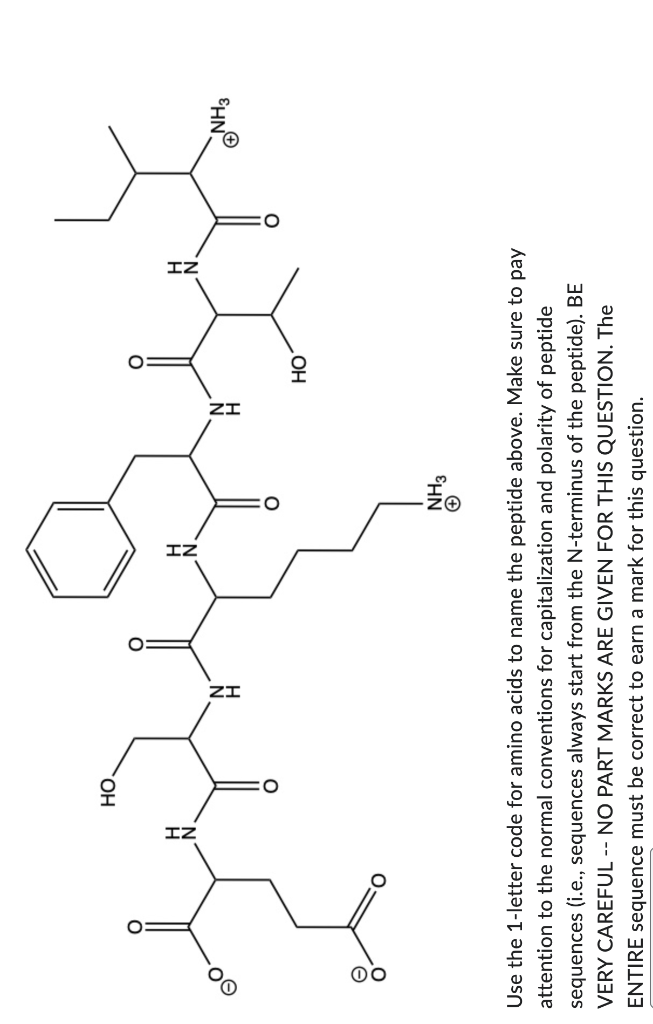
You have a mixture of 4 amino acids (Q,W,E,R,T) that you want to separate using ion exchange chromatography. Which of these amino acids would bind most strongly to a CM cation exchange column at pH 7? Q W E R T Which of the followIng methods would be expected to help elute (I.e., WEAKEN the binding of) proteins from a CATION EXCHANGE column with carboxymethyl groups in the stationary phase and a mobile phase with pH=6.0. Each correctly assigned choice (i.e., either checked or not) earns 0.25 marks. Each incorrectly assigned choice earns 0 marks. Increasing the concentration of salt (NaCl) in the mobile phase from 0.1M to 0.5 M for a protein with pl=7.0. Decreasing the pH of the mobile phase from 6.0 to 5.0 for a protein with pl= 6.5 Increasing the pH of the mobile phase from 6.0 to 7.0 for a protein with pl=6.5. Increasing the pH of the mobile phase from 6.0 to 7.0AND increasing the concentration of salt (NaCl) from 0.1M to 0.5M for a protein with pl=7.5. Use the 1-letter code for amino acids to name the peptide above. Make sure to pay attention to the normal conventions for capitalization and polarity of peptide sequences (i.e., sequences always start from the N-terminus of the peptide). BE VERY CAREFUL -- NO PART MARKS ARE GIVEN FOR THIS QUESTION. The ENTIRE sequence must be correct to earn a mark for thisStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


