Answered step by step
Verified Expert Solution
Question
1 Approved Answer
pls answer this ASAP 22. Aldehydes and ketones are common air pollutants produced by oxidation of hydrocarbons released from gasoline engines. A student sampled 100
pls answer this ASAP 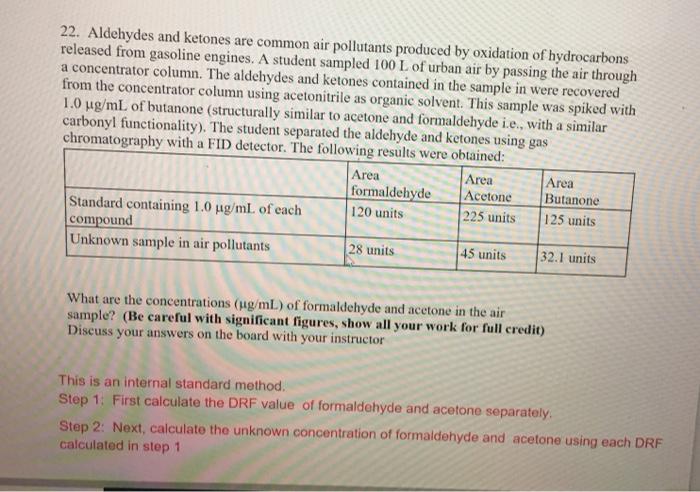
22. Aldehydes and ketones are common air pollutants produced by oxidation of hydrocarbons released from gasoline engines. A student sampled 100 L of urban air by passing the air through a concentrator column. The aldehydes and ketones contained in the sample in were recovered from the concentrator column using acetonitrile as organic solvent. This sample was spiked with 1.0 ug/mL of butanone (structurally similar to acetone and formaldehyde i.e., with a similar carbonyl functionality). The student separated the aldehyde and ketones using gas chromatography with a FID detector. The following results were obtained: Area Area Area formaldehyde Acetone Butanone Standard containing 1.0 ug/mL of each 120 units 225 units 125 units compound Unknown sample in air pollutants 28 units 45 units 32.1 units What are the concentrations (g/mL) of formaldehyde and acetone in the air sample? (Be careful with significant figures, show all your work for full credit) Discuss your answers on the board with your instructor This is an internal standard method. Step 1: First calculate the DRF value of formaldehyde and acetone separately. Step 2: Next, calculate the unknown concentration of formaldehyde and acetone using each DRF calculated in step 1 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


