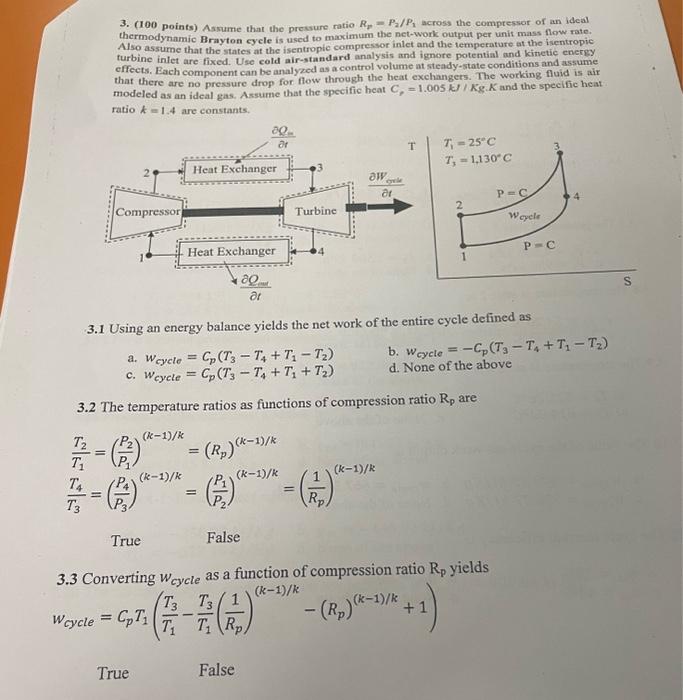
pls answr from 3.4 onward until you are allowed to answer

3.12 For a constant pressure and quasi-static process, the specific enthalpy of state 1 is a. h1=299.49kJ/Kg c. h1=278.98kf/Kg b. h1=345.76kJ/Kg d. None of the above 3.13 The temperature of the state 2 is a. T2=373C c. T2=264C b. T2=471C d. None of the above 3.14 For a constant pressure and quasi-static process, the specific enthalpy of state 2 is a. h2=649.23kJ/Kg c. h2=456.12kJ/Kg b. h2=299.56kJ/Kg d. None of the above 3.15 For a constant pressure and quasi-static process, the specific enthalpy of the state 3 is a. h3=1410.02kJ/Kg b. h3=1432.46kJ/Kg c. h2=1365.78kJ/Kg d. None of the above 3.16 The temperature of the state 4 is a. T4=374.19C b. T4=471.50C c. T4=264.67C d. None of the above 3.17 For a constant pressure and quasi-static process, the specific enthalpy of the state 4 is a. h4=650.43kJ/Kg b. h4=912.34kJ/Kg c. h4=765.82kJ/Kg d. None of the above 3.18 Process 2-3: Isobaric heat addition is a. q23=760.82kJ/Kg b. q23=1045.23kJ/Kg c. q23=1245.87kJ/Kg d. None of the above 3.19 Process 4-1: Isobaric heat rejection is a. q41=350.94kJ/Kg b. q41=445.92kJ/Kg c. q41=623.15kJ/Kg d. None of the above 3.20 The calculated cycle work based on temperatures is wcycte409.84kJ/Kg True False 3. (100 points) Aspume that the pressure ratio Rp=P2/P1 across the comptessor of an ideal thermodynamic Brayton cycle is used to maximum the net-work output per unit mass flow rate. Also assume that the states at the isentropic compressor inlet and the temperature at the isentropie turbine inlet are fixed. Use cold air-standard analysis and ignore potential and kinetic energy effects. Bach component can be analyzed as a control volume at steady-state conditions and assume that there are no pressure drop for flow through the heat exehangers. The working fluid is air modeled as an ideal gas. Assume that the specific heat Cp=1.005kf/KgK and the speeific heat ratio k=1.4 are constants. 3.1 Using an energy balance yields the net work of the entire cycle defined as a. wcycle=Cp(T3T4+T1T2) b. wcycle=Cp(T3T4+T1T2) c. wcycle=Cp(T3T4+T1+T2) d. None of the above 3.2 The temperature ratios as functions of compression ratio Rp are T1T2=(P1P2)(k1)/k=(Rp)(k1)/kT3T4=(P3P4)(k1)/k=(P2P1)(k1)/k=(Rp1)(k1)/k True False 3.3 Converting wcycle as a function of compression ratio Rp yields wcycle=CpT1(T1T3T1T3(Rp1)(k1)/k(Rp)(k1)/k+1) 3.4 The derivative of WcycleasafunctionofcompressionratioR,is Rpwcycie=CpT1[T1T3k1(k1)(Rp)[(1k1)/k]1k1(k1)(Rp)[(k1)/k]1] True False 3.5 The compression ratio is a. Rp=15 b. Rp=28 c. Rp=7 d. None of the above 3.6 The pressure at state 2 is a. P2=1,500kPa b. P2=2,500kPa c. P2=980kPa d. None of the above 3. 7 The pressure at state 3 is a. P3=1,500kPa b. P3=2,500kPa c. P3=980kPa d. None of the above 3.8 The temperature at state 2 is a. T2=54.20C b. T2=66.31C c. T2=74.93C d. None of the above 3.9 The temperature at state 4 is a. T4=521.26C b. T4=732.66C c. T4=614.82C d. None of the above 3.10 The value of the cycle net-work output is a. wcycte=409.83kJ/Kg b. wcycle=667.54kJ/Kg c. wcycle=556.77kJ/Kg d. None of the above 3.11 The cycle thermal efficiency is a. nth=38% b. nth=50% c. neh=44%6 d. None of the above 3.12 For a constant pressure and quasi-static process, the specific enthalpy of state 1 is a. h1=299.49kJ/Kg c. h1=278.98kf/Kg b. h1=345.76kJ/Kg d. None of the above 3.13 The temperature of the state 2 is a. T2=373C c. T2=264C b. T2=471C d. None of the above 3.14 For a constant pressure and quasi-static process, the specific enthalpy of state 2 is a. h2=649.23kJ/Kg c. h2=456.12kJ/Kg b. h2=299.56kJ/Kg d. None of the above 3.15 For a constant pressure and quasi-static process, the specific enthalpy of the state 3 is a. h3=1410.02kJ/Kg b. h3=1432.46kJ/Kg c. h2=1365.78kJ/Kg d. None of the above 3.16 The temperature of the state 4 is a. T4=374.19C b. T4=471.50C c. T4=264.67C d. None of the above 3.17 For a constant pressure and quasi-static process, the specific enthalpy of the state 4 is a. h4=650.43kJ/Kg b. h4=912.34kJ/Kg c. h4=765.82kJ/Kg d. None of the above 3.18 Process 2-3: Isobaric heat addition is a. q23=760.82kJ/Kg b. q23=1045.23kJ/Kg c. q23=1245.87kJ/Kg d. None of the above 3.19 Process 4-1: Isobaric heat rejection is a. q41=350.94kJ/Kg b. q41=445.92kJ/Kg c. q41=623.15kJ/Kg d. None of the above 3.20 The calculated cycle work based on temperatures is wcycte409.84kJ/Kg True False 3. (100 points) Aspume that the pressure ratio Rp=P2/P1 across the comptessor of an ideal thermodynamic Brayton cycle is used to maximum the net-work output per unit mass flow rate. Also assume that the states at the isentropic compressor inlet and the temperature at the isentropie turbine inlet are fixed. Use cold air-standard analysis and ignore potential and kinetic energy effects. Bach component can be analyzed as a control volume at steady-state conditions and assume that there are no pressure drop for flow through the heat exehangers. The working fluid is air modeled as an ideal gas. Assume that the specific heat Cp=1.005kf/KgK and the speeific heat ratio k=1.4 are constants. 3.1 Using an energy balance yields the net work of the entire cycle defined as a. wcycle=Cp(T3T4+T1T2) b. wcycle=Cp(T3T4+T1T2) c. wcycle=Cp(T3T4+T1+T2) d. None of the above 3.2 The temperature ratios as functions of compression ratio Rp are T1T2=(P1P2)(k1)/k=(Rp)(k1)/kT3T4=(P3P4)(k1)/k=(P2P1)(k1)/k=(Rp1)(k1)/k True False 3.3 Converting wcycle as a function of compression ratio Rp yields wcycle=CpT1(T1T3T1T3(Rp1)(k1)/k(Rp)(k1)/k+1) 3.4 The derivative of WcycleasafunctionofcompressionratioR,is Rpwcycie=CpT1[T1T3k1(k1)(Rp)[(1k1)/k]1k1(k1)(Rp)[(k1)/k]1] True False 3.5 The compression ratio is a. Rp=15 b. Rp=28 c. Rp=7 d. None of the above 3.6 The pressure at state 2 is a. P2=1,500kPa b. P2=2,500kPa c. P2=980kPa d. None of the above 3. 7 The pressure at state 3 is a. P3=1,500kPa b. P3=2,500kPa c. P3=980kPa d. None of the above 3.8 The temperature at state 2 is a. T2=54.20C b. T2=66.31C c. T2=74.93C d. None of the above 3.9 The temperature at state 4 is a. T4=521.26C b. T4=732.66C c. T4=614.82C d. None of the above 3.10 The value of the cycle net-work output is a. wcycte=409.83kJ/Kg b. wcycle=667.54kJ/Kg c. wcycle=556.77kJ/Kg d. None of the above 3.11 The cycle thermal efficiency is a. nth=38% b. nth=50% c. neh=44%6 d. None of the above