Answered step by step
Verified Expert Solution
Question
1 Approved Answer
pls help ill thumbs up A student obtained the following data for the decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30 C. N2052
pls help ill thumbs up 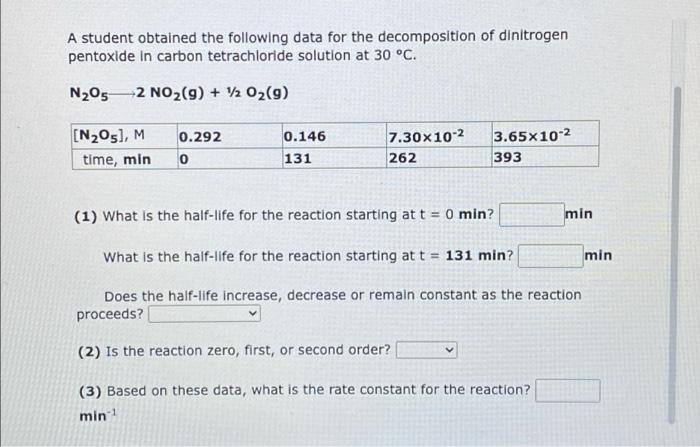
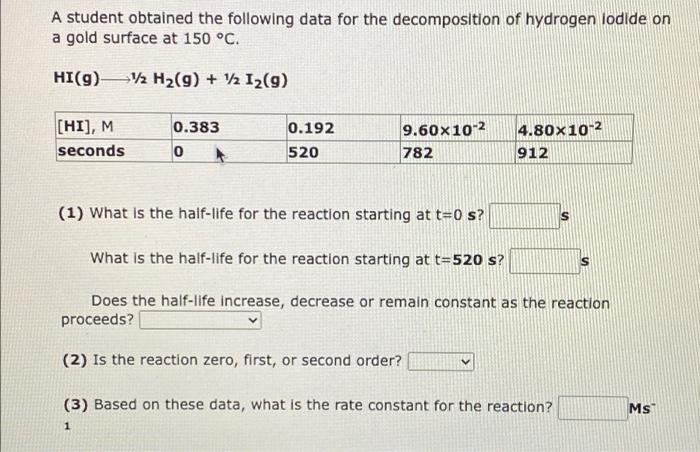
A student obtained the following data for the decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30 C. N2052 NO2(g) + V2 O2(9) [N205), M time, min 0.292 0 0.146 131 7.30X10-2 262 3.65x10-2 393 (1) What is the half-life for the reaction starting at t = 0 min? min What is the half-life for the reaction starting at t = 131 min? min Does the half-life increase, decrease or remain constant as the reaction proceeds? (2) Is the reaction zero, first, or second order? (3) Based on these data, what is the rate constant for the reaction? min A student obtained the following data for the decomposition of hydrogen lodide on a gold surface at 150 C. HI(g)12 H2(9) + 12 12(9) 0.383 [HI], M seconds 0.192 520 9.60x10-2 4.80x10-2 912 0 782 (1) What is the half-life for the reaction starting at t=0 s? What is the half-life for the reaction starting at t=520 s? S Does the half-life increase, decrease or remain constant as the reaction proceeds? (2) Is the reaction zero, first, or second order? Ms (3) Based on these data, what is the rate constant for the reaction? 1 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


