Answered step by step
Verified Expert Solution
Question
1 Approved Answer
pls help me to solve this questions 2- A refinery stack emits gas mixture of 15%CO2,10%CO,5%H2,35%N2, 10%O2 and the balance H2O (in mole %). The
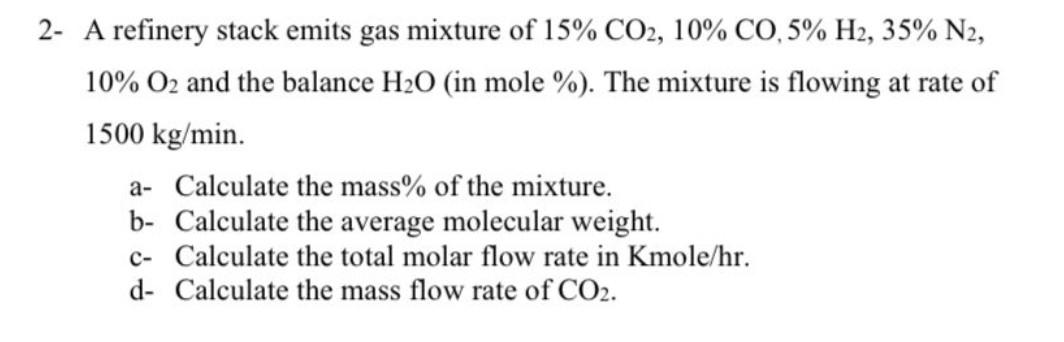

pls help me to solve this questions
2- A refinery stack emits gas mixture of 15%CO2,10%CO,5%H2,35%N2, 10%O2 and the balance H2O (in mole \%). The mixture is flowing at rate of 1500kg/min. a- Calculate the mass % of the mixture. b- Calculate the average molecular weight. c- Calculate the total molar flow rate in Kmole/hr. d- Calculate the mass flow rate of CO2. - A coal combustion batch reactor produces CO2 as a product. How of each of the following are contained in 100.0g of CO2 (molecular weight (MW)= 44g/gmole )? (1) mole CO2; (2) lb-moles CO2; (3) mole C; (4) mole O; (5) mole O2; (6) gO2; (7) molecules of CO2Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


