Answered step by step
Verified Expert Solution
Question
1 Approved Answer
pls help! Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, and hydrogen prepared by reforming natural gas, in a two-step process.
pls help! 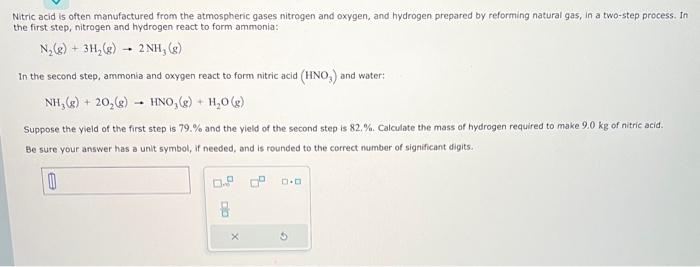
Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, and hydrogen prepared by reforming natural gas, in a two-step process. In the first step, nitrogen and hydrogen react to form ammonia: N2(g)+3H2(g)2NH3(g) In the second step, ammonia and oxygen react to form nitric acid (HNO3) and water: NH3(g)+2O2(g)HNO3(g)+H2O(g) Suppose the yield of the first step is 79.% and the yield of the second step is 82,%. Calculate the mass of hydrogen required to make 9.0kg of nitric acid. Be sure your answer has a unit symbol, if needed, and is rounded to the correct number of significant digits 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


