Answered step by step
Verified Expert Solution
Question
1 Approved Answer
pls urgent 1.87gH2 is allowed to react with 9.75gN2, producing 1.36gNH3. Part A What is the theoretical yield in grams for this reaction under the
pls urgent 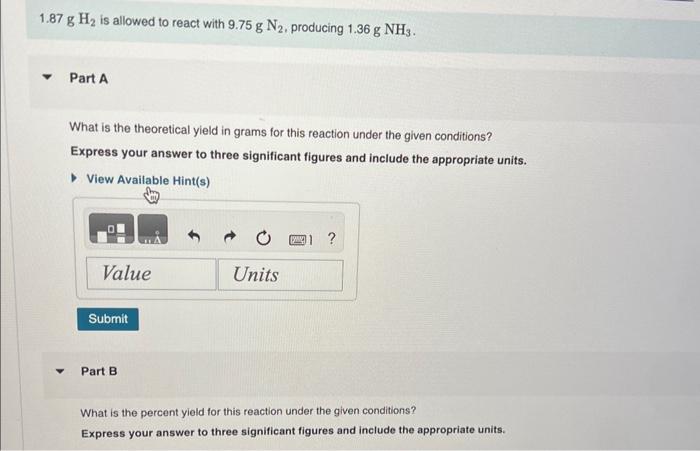
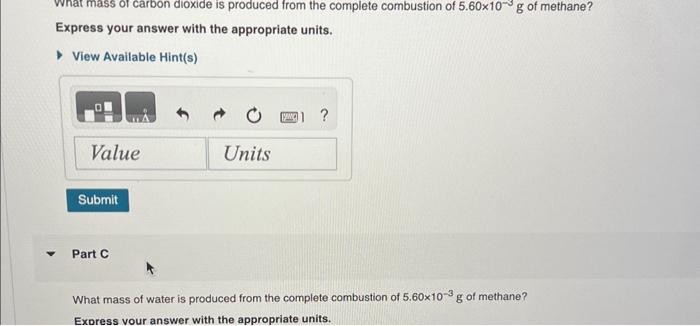
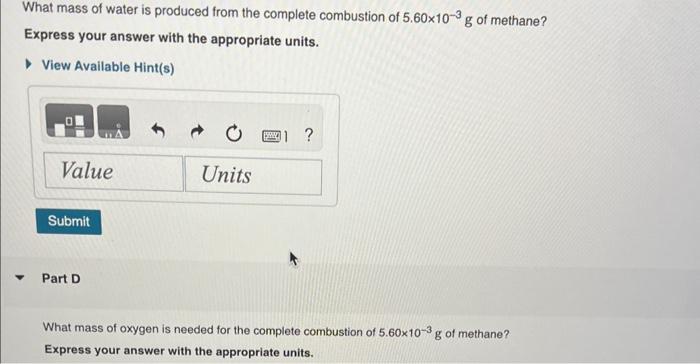
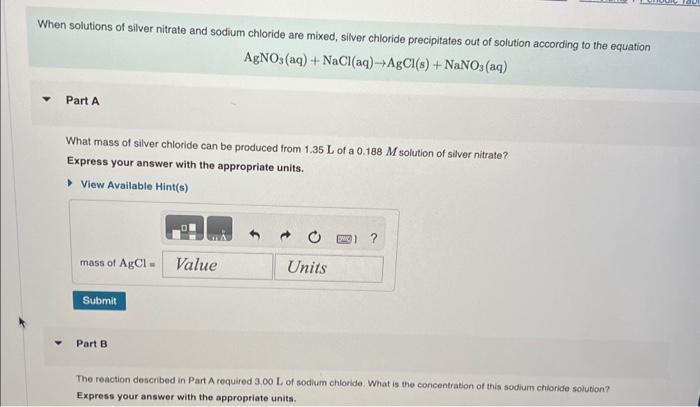


1.87gH2 is allowed to react with 9.75gN2, producing 1.36gNH3. Part A What is the theoretical yield in grams for this reaction under the given conditions? Express your answer to three significant figures and include the appropriate units. View Available Hint(s) Part B What is the percent yield for this reaction under the given conditions? Express your answer to three significant figures and include the appropriate units. What mass of carbon dioxide is produced from the complete combustion of 5.60103g of methane? Express your answer with the appropriate units. View Available Hint(s) Part C What mass of water is produced from the complete combustion of 5.60103g of methane? Express vour answer with the appropriate units. What mass of water is produced from the complete combustion of 5.60103g of methane? Express your answer with the appropriate units. Part D What mass of oxygen is needed for the complete combustion of 5.60103g of methane? Express your answer with the appropriate units. When solutions of silver nitrate and sodium chloride are mixed, silver chloride precipitates out of solution according to the equation AgNO3(aq)+NaCl(aq)AgCl(s)+NaNO3(aq) Part A What mass of silver chloride can be produced from 1.35 L of a 0.188M solution of silver nitrate? Express your answer with the appropriate units. Part B The reaction described in Part A required 3.00L of sodium chloride. What is the concentration of this sodium chioride solution? Express your answer with the appropriate units. ydrochloric acid ( HCl) reacts with sodium carbonate (Na2CO3), forming sodium chloride (NaCl), water (H2O), and carbon dioxide CO2 ). This equation is balanced as written: 2HCl(aq)+Na2CO3(aq)2NaCl(aq)+H2O(l)+CO2(g) PartA ? Express your answer numerically in litres. A e49-mL sample of unknown HCl solution reacts completely with Na2CO3 to form 16.1gCO2. What was the concentration of the HCl solution? Express the molor concentration numerically 





Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


