Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PLSS STEP BY STEP SOLUTION! :) The first chemical step in making sulfuric acid from elemental sulfur is burning the sulfur (completely) to form SO2.
PLSS STEP BY STEP SOLUTION! :)
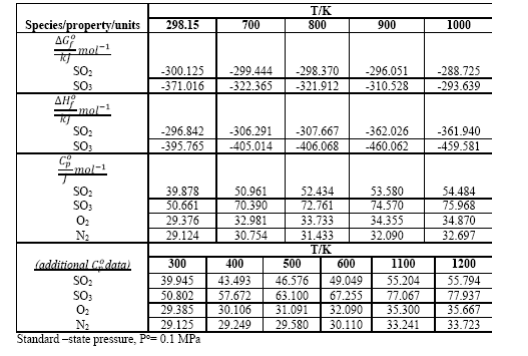
The first chemical step in making sulfuric acid from elemental sulfur is burning the sulfur (completely) to form SO2. Calculate the temperature of the gas leaving the burner based on the following: the burner operates adiabatically, sulfur enters as liquid at 140C, and excess air (79% mol N2, 21% O2) enters at 25C; H (S(), 140C S(s,rh), 25C) = -5.07 kJmol-1; the outlet gas contains 9.5 mol % SO2; other data required are given in table.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


