Answered step by step
Verified Expert Solution
Question
1 Approved Answer
plz ans soon as possible (d) If inverse interpolation is appropriate, estimate the value of P when enthalpy of evaporation (hfg) = 1776 kJ/kg based

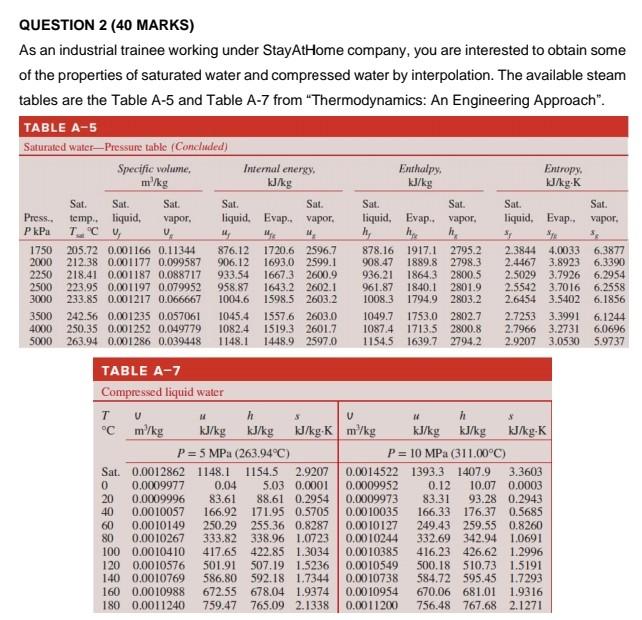
plz ans soon as possible
(d) If inverse interpolation is appropriate, estimate the value of P when enthalpy of evaporation (hfg) = 1776 kJ/kg based on the data between 2500 - 3500 kPa. (7 Marks) 3213.0169 kPa QUESTION 2 (40 MARKS) As an industrial trainee working under StayAtHome company, you are interested to obtain some of the properties of saturated water and compressed water by interpolation. The available steam tables are the Table A-5 and Table A-7 from "Thermodynamics: An Engineering Approach". TABLE A-5 Saturated water Pressure table (Concluded) Specific volume. Internal energy Enthalpy: Entropy. m/kg kJ/kg J/kg IJ/ky K Sat. Sat. Sat. Sat. Sat. Sat. Sat. Sat. Sat. Press. temp.. liquid, vapor. liquid. Evap.. vapor. liquidEvap.. vapor. liquid, Evap.. vapor. Pk TCU V Me W hy h 3 3: 1750 205.72 0.001166 0.11344 876.12 1720.6 2596.7 878.16 1917.1 2795.2 2.3844 4.0033 6.3877 2000 212.38 0.001177 0.099587 906.12 1693.0 2599.1 908.47 1889.8 2798.3 2.4467 3.8923 6.3390 2250 218.41 0.001187 0.088717 933.54 1667.3 2600.9 936.21 1864.3 2800.5 2.5029 3.7926 6.2954 2500 223.95 0.001197 0.079952 958.87 1643.2 2602.1 961.87 1840.1 2801.9 2.5542 3.7016 6.2558 3000 233.85 0.001217 0.066667 1004.6 1598.5 2603.2 1008.3 1794.9 2803.2 2.6454 3.5402 6.1856 3500 242.56 0.001235 0.057061 1045.4 1557.6 2603.0 1049.7 1753.0 2802.7 2.7253 3.3991 6.1244 4000 250.35 0.001252 0.049779 1082.4 1519.3 2601.7 1087.4 1713.5 2800.8 2.7966 3.2731 6,0696 5000 263.94 0.001286 0.039448 1148.1 1448.9 2597.0 1154.5 1639.7 2794.2 2.9207 3,0530 5.9737 TABLE A-7 Compressed liquid water Tv h 5 U h 3 Cm/kg kJ/kg kJ/kg IJ/kg- Km/kg kJ/kg kJ/kg kJ/kg.K P= 5 MPa (263.94C) P= 10 MPa (311.00C) Sat. 0,0012862 1148.1 1154.5 2.9207 0.0014522 1393.3 1407.9 3.3603 0 0.0009977 0.04 5.03 0.0001 0.0009952 0.12 10.07 0.0003 20 0.0009996 83.61 88.61 0.2954 0.0009973 83.31 93.28 0.2943 40 0.0010057 166.92 171.95 0.5705 0.0010035 166.33 176,37 0.5685 60 0.0010149 250.29 255.36 0.8287 0.0010127 249.43 259.55 0.8260 80 0.0010267 333.82 338.96 1.0723 0.0010244 332.69 342.94 1.0691 100 0.0010410 417.65 422.85 1.3034 0.0010385 416.23 426,62 1.2996 120 0.00 10576 501.91 507.19 1.5236 0.0010549 500.18 510.73 1.5191 140 0.0010769 586.80 592.18 1.7344 0.0010738 584.72 595.45 1.7293 160 0.0010988 672.55 678.04 1.9374 0.0010954 670.06 681.01 1.9316 180 0.0011240 759.47 765.09 2.1338 0.0011200 756.48 767.68 2.1271Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


