Answered step by step
Verified Expert Solution
Question
1 Approved Answer
plz answer all question 1. Given the following reaction: N,05 (g) -NO2 (g) + O2(g) 1. Write the rate if reaction in terms of the
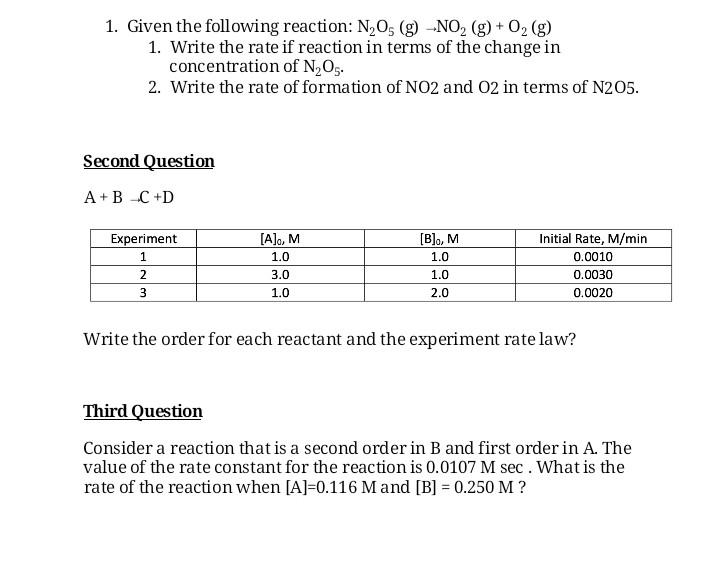
plz answer all question
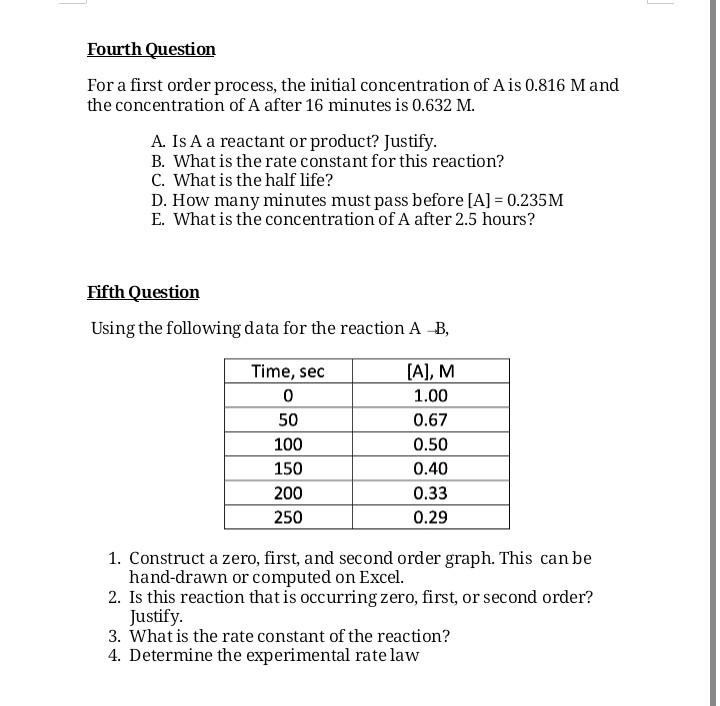
1. Given the following reaction: N,05 (g) -NO2 (g) + O2(g) 1. Write the rate if reaction in terms of the change in concentration of N,O.. 2. Write the rate of formation of NO2 and 02 in terms of N205. Second Question A+B C +D Experiment 1 2 3 [A]o, M 1.0 3.0 1.0 [B], M 1.0 1.0 2.0 Initial Rate, M/min 0.0010 0.0030 0.0020 Write the order for each reactant and the experiment rate law? Third Question Consider a reaction that is a second order in B and first order in A. The value of the rate constant for the reaction is 0.0107 M sec . What is the rate of the reaction when [A]=0.116 Mand [B] = 0.250 M ? Fourth Question For a first order process, the initial concentration of Ais 0.816 Mand the concentration of A after 16 minutes is 0.632 M. A. Is A a reactant or product? Justify. B. What is the rate constant for this reaction? C. What is the half life? D. How many minutes must pass before [A] = 0.235M E. What is the concentration of A after 2.5 hours? Fifth Question Using the following data for the reaction A B, Time, sec 0 50 100 150 200 250 [A], M 1.00 0.67 0.50 0.40 0.33 0.29 1. Construct a zero, first, and second order graph. This can be hand-drawn or computed on Excel. 2. Is this reaction that is occurring zero, first, or second order? Justify. 3. What is the rate constant of the reaction? 4. Determine the experimental rate lawStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


