Answered step by step
Verified Expert Solution
Question
1 Approved Answer
plz asap solve withh full ans Question 1 a) Clapeyron equation is widely used to determine the enthalpy/entropy change of vaporization. For 1-butene it determines
plz asap solve withh full ans 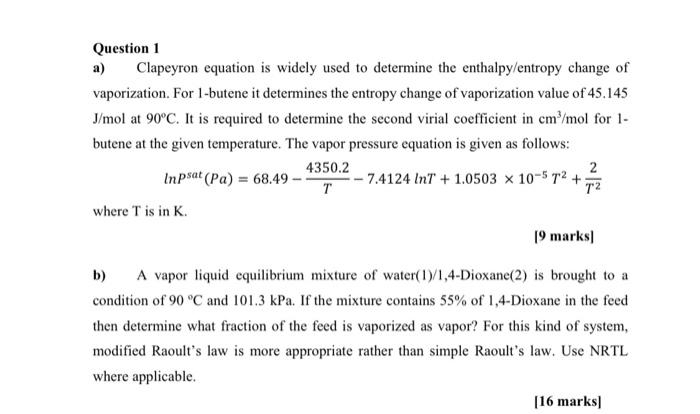
Question 1 a) Clapeyron equation is widely used to determine the enthalpy/entropy change of vaporization. For 1-butene it determines the entropy change of vaporization value of 45.145 J/mol at 90C. It is required to determine the second virial coefficient in cm/mol for 1- butene at the given temperature. The vapor pressure equation is given as follows: 4350.2 2 Inpsat (Pa) = 68.49 - 7.4124 InT + 1.0503 x 10-5 12 + 12 where T is in K. 19 marks) b) A vapor liquid equilibrium mixture of water(19/1,4-Dioxane(2) is brought to a condition of 90 C and 101.3 kPa. If the mixture contains 55% of 1,4-Dioxane in the feed then determine what fraction of the feed is vaporized as vapor? For this kind of system, modified Raoult's law is more appropriate rather than simple Raoult's law. Use NRTL where applicable [16 marks) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


