Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PLZ HELP ASAP 1. A) Define amphipathic molecules using drawings and complete sentences (refer to p139 of the textbook). 2 B) Identify the polar and
PLZ HELP ASAP 
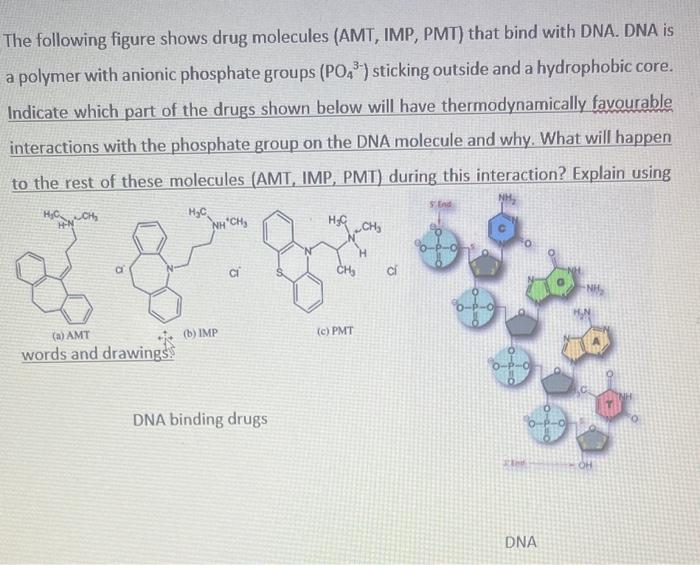

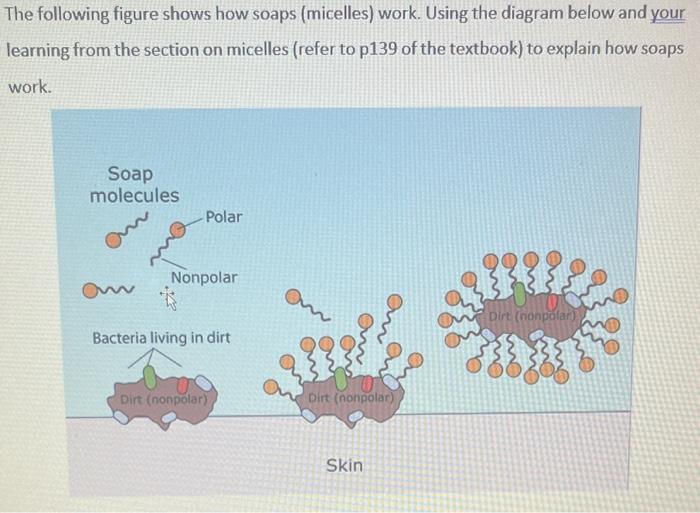
1. A) Define amphipathic molecules using drawings and complete sentences (refer to p139 of the textbook). 2 B) Identify the polar and non-polar parts of this amphipathic molecule. Explain the basis of these identifications based on electronic properties of elements (refer to 1139 from the textbook) The following figure shows drug molecules (AMT, IMP, PMT) that bind with DNA. DNA is a polymer with anionic phosphate groups (PO43) sticking outside and a hydrophobic core. Indicate which part of the drugs shown below will have thermodynamically favourable interactions with the phosphate group on the DNA molecule and why. What will happen to the rest of these molecules (AMT, IMP, PMT) during this interaction? Explain using words and drawingss The following figure shows the structure of a peptide (made of amino acids). Peptides and proteins are like ribbons and can fold on itself forming a 3D structure. Pay attention to the functional groups in the boxed areas. When this solid peptide is added to water, identify A) the functional groups that will dissolve in water, and B) the functional groups that will not dissolve in water. Explain the contribution of S and H towards the spontaneous folding of this peptide. The following figure shows how soaps (micelles) work. Using the diagram below and your learning from the section on micelles (refer to 139 of the textbook) to explain how soaps work. 1. A) Define amphipathic molecules using drawings and complete sentences (refer to p139 of the textbook). 2 B) Identify the polar and non-polar parts of this amphipathic molecule. Explain the basis of these identifications based on electronic properties of elements (refer to 1139 from the textbook) The following figure shows drug molecules (AMT, IMP, PMT) that bind with DNA. DNA is a polymer with anionic phosphate groups (PO43) sticking outside and a hydrophobic core. Indicate which part of the drugs shown below will have thermodynamically favourable interactions with the phosphate group on the DNA molecule and why. What will happen to the rest of these molecules (AMT, IMP, PMT) during this interaction? Explain using words and drawingss The following figure shows the structure of a peptide (made of amino acids). Peptides and proteins are like ribbons and can fold on itself forming a 3D structure. Pay attention to the functional groups in the boxed areas. When this solid peptide is added to water, identify A) the functional groups that will dissolve in water, and B) the functional groups that will not dissolve in water. Explain the contribution of S and H towards the spontaneous folding of this peptide. The following figure shows how soaps (micelles) work. Using the diagram below and your learning from the section on micelles (refer to 139 of the textbook) to explain how soaps work 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


