Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Plz show your process (DOn't answer like $Delta H_{r}=-165.01 mathrm{kJ/mol}$!!! Public concern about the increase in CO2 in the atmosphere has led to many proposals
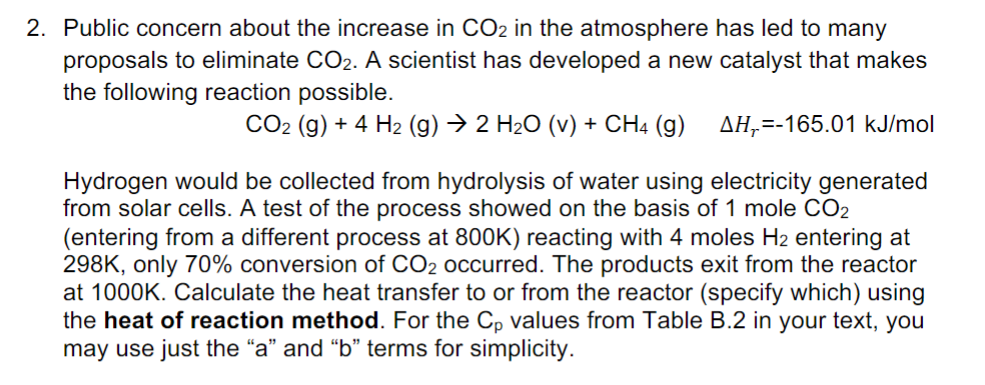
Plz show your process (DOn't answer like "$\Delta H_{r}=-165.01\ \mathrm{kJ/mol}$"!!!
Public concern about the increase in CO2 in the atmosphere has led to many proposals to eliminate CO2. A scientist has developed a new catalyst that makes the following reaction possible. CO2(g)+4H2(g)2H2O(v)+CH4(g)Hr=165.01kJ/mol Hydrogen would be collected from hydrolysis of water using electricity generated from solar cells. A test of the process showed on the basis of 1 mole CO2 (entering from a different process at 800K ) reacting with 4 moles H2 entering at 298K, only 70% conversion of CO2 occurred. The products exit from the reactor at 1000K. Calculate the heat transfer to or from the reactor (specify which) using the heat of reaction method. For the Cp values from Table B.2 in your text, you may use just the "a" and "b" terms for simplicity
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


