Answered step by step
Verified Expert Solution
Question
1 Approved Answer
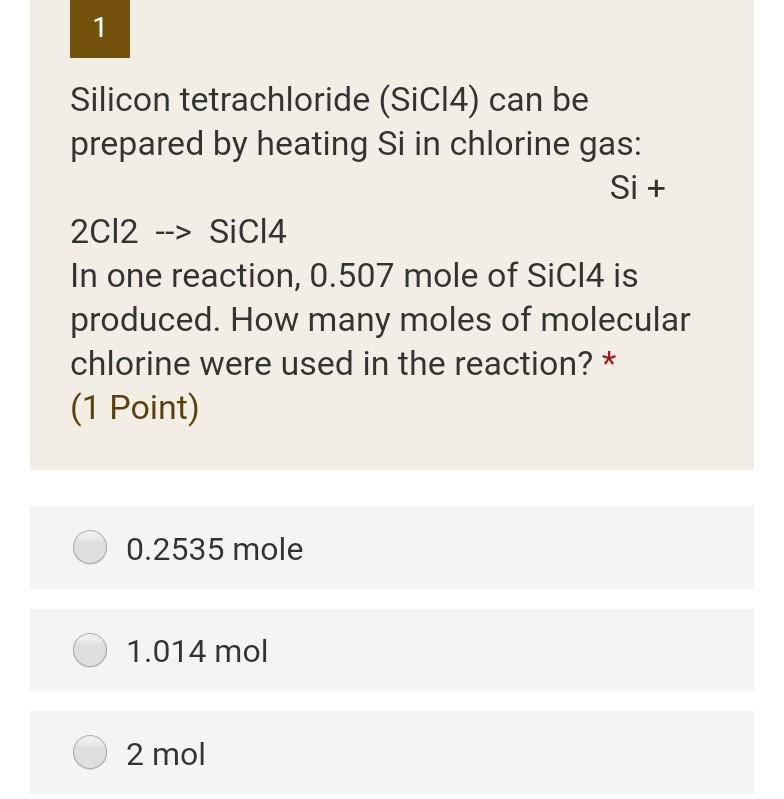
Plzzzzzz 1 Silicon tetrachloride (SiCl4) can be prepared by heating Si in chlorine gas: Si + 2012 --> SiCl4 In one reaction, 0.507 mole of


Plzzzzzz
1 Silicon tetrachloride (SiCl4) can be prepared by heating Si in chlorine gas: Si + 2012 --> SiCl4 In one reaction, 0.507 mole of SiCl4 is produced. How many moles of molecular chlorine were used in the reaction? * (1 Point) 0.2535 mole 1.014 mol 2 mol 2 Certain race cars use methanol (CH3OH, also called wood alcohol) as a fuel. The combustion of methanol occurs according to the following equation: 2CH3OH + 302 --> 2002 + 4H20 In a particular reaction, 9.8 moles of CH3OH are reacted with an excess of 02. Calculate the number of moles of H20 formed. * (2 Points) 19.6 mol 4.9 mol 9.8 mol 3 How many grams of sulfur (S) are needed to react completely with 246 g of mercury g (Hg) to form HgS? * (2 Points) 1.23 g 39.367 g 1537.24 gStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


