Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Polypropylene (PP) is a hydrocarbon polymer produced from the reaction of chemicals obtained from crude oil. PP is used in many applications including the

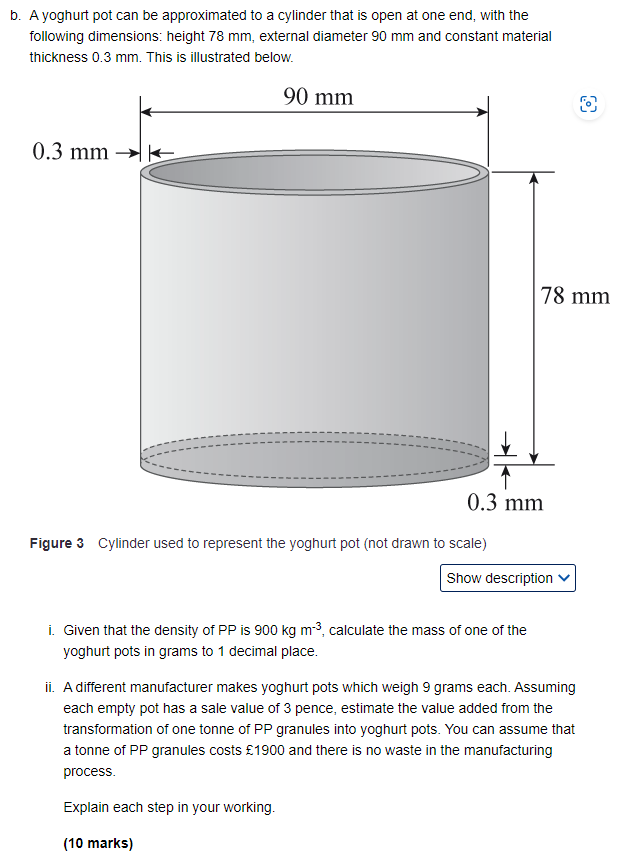

Polypropylene (PP) is a hydrocarbon polymer produced from the reaction of chemicals obtained from crude oil. PP is used in many applications including the manufacture of food containers such as yoghurt pots. Like plastic bags (covered in section 16.2.1 in Part 4 'Materials and resources'), PP containers present challenges to the engineering community when they reach the end of their lives. PP containers can be re-used, and some manufacturers encourage this. However, large quantities are discarded as waste each year. Two possible disposal options are: PP can be recycled into new containers or other products. PP releases a lot of energy when burned, so it is a useful potential fuel for waste fired power stations (see Activity 8.1a) along with other components of the waste stream. (Apart from the greenhouse gas CO2, PP does not release harmful chemicals into the atmosphere when burned). a. In Chapter 14, Activity 14.15, you were asked to list the activities involved in producing a can of baked beans and getting it to market. For this question, make a similar list to cover each process in the chain of activities needed to produce a PP container filled with plain yoghurt, from the raw materials to the point of purchase by the customer. The first three stages of production have been provided below for you. You are not expected to include details of the manufacturing method. Continue the list of bullet-points with another 8 suggestions to cover the entire range of activities to the point of sale to the customer. Each stage should be one or two sentences long. Extraction of crude oil from the ground. Refining of the crude oil and production of chemicals needed to produce PP. Production of PP and processing into pellets. (4 marks) b. A yoghurt pot can be approximated to a cylinder that is open at one end, with the following dimensions: height 78 mm, external diameter 90 mm and constant material thickness 0.3 mm. This is illustrated below. 0.3 mm K 90 mm 8 78 mm 0.3 mm Figure 3 Cylinder used to represent the yoghurt pot (not drawn to scale) Show description i. Given that the density of PP is 900 kg m, calculate the mass of one of the yoghurt pots in grams to 1 decimal place. ii. A different manufacturer makes yoghurt pots which weigh 9 grams each. Assuming each empty pot has a sale value of 3 pence, estimate the value added from the transformation of one tonne of PP granules into yoghurt pots. You can assume that a tonne of PP granules costs 1900 and there is no waste in the manufacturing process. Explain each step in your working. (10 marks) c. As an alternative, yoghurt is occasionally sold in glass jars. Compare the two materials and give two advantages and two disadvantages for using glass rather than PP. (10 marks) d. The chemical formula for one repeat unit of the PP polymer is C3H6. Write down a balanced chemical equation for the complete combustion of one repeat unit of PP in oxygen to form carbon dioxide and water (you may find it helpful to show the steps in your working as described in Section 6.2, Part 2 'Engineering for power'). Explain how you can test that your equation is balanced. (6 marks) e. The heat of combustion of PP is 38.7 MJ kg-1. If PP containers are burned in a waste- fired power station that generates electricity with an overall efficiency of 19%, calculate how much useful electrical energy is generated when 1,000 tonnes of PP is burned. Give your answer in units of MJ to 3 significant figures and explain your working.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


