Answered step by step
Verified Expert Solution
Question
1 Approved Answer
polyymer chemistry and engineering Can you solve it in detail solutions 2. Consider the polymerization of methyl methacrylate to produce PMMA. Given the data below,
polyymer chemistry and engineering 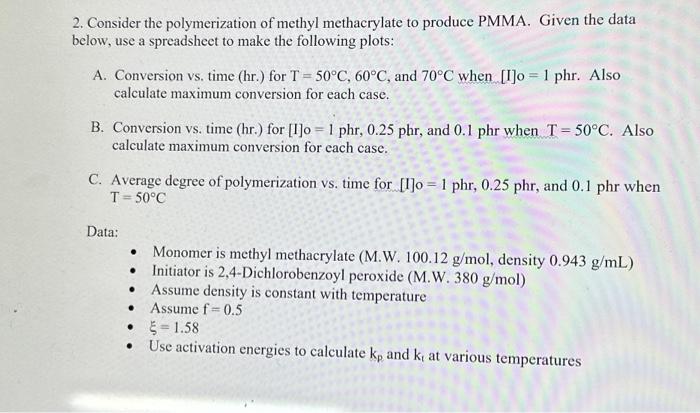
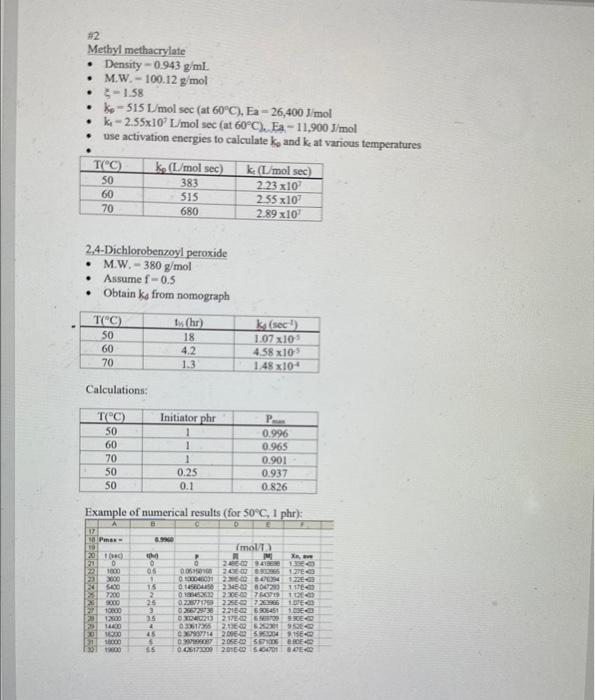
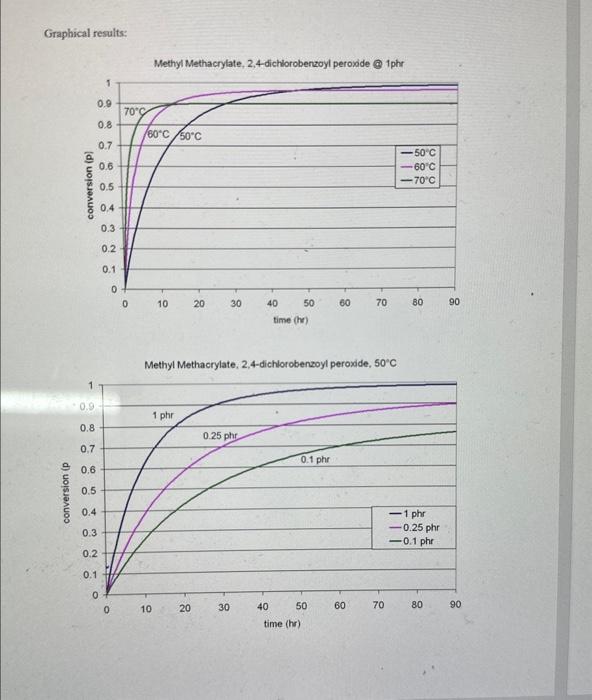
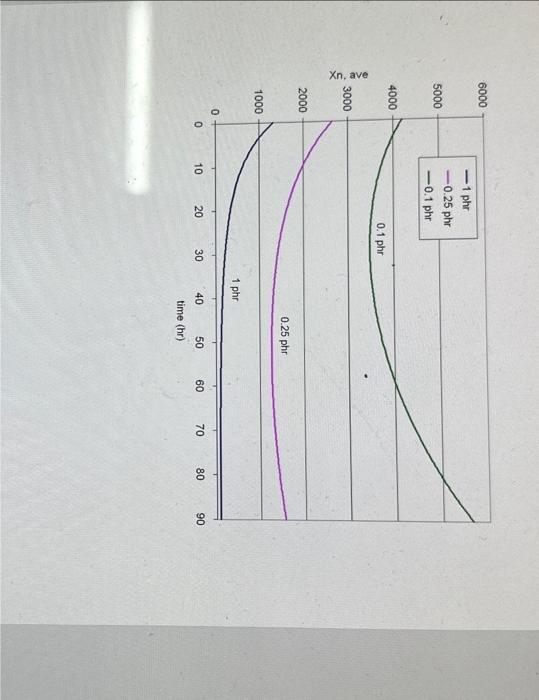
2. Consider the polymerization of methyl methacrylate to produce PMMA. Given the data below, use a spreadsheet to make the following plots: A. Conversion vs, time (hr.) for T=50C,60C, and 70C when [I]o=1 phr. Also calculate maximum conversion for each case. B. Conversion vs. time (hr.) for [I]o=1phr,0.25phr, and 0.1 phr when T=50C. Also calculate maximum conversion for each case. C. Average degree of polymerization vs. time for [1]o=1phr,0.25phr, and 0.1phr when T=50C Data: - Monomer is methyl methacrylate (M.W. 100.12g/mol, density 0.943g/mL ) - Initiator is 2,4-Dichlorobenzoyl peroxide (M.W. 380g/mol) - Assume density is constant with temperature - Assume f=0.5 - =1.58 - Use activation energies to calculate kp and kt at various temperatures 2 Methyl methacrylate - Density =0.943g/mL - M.W. 100.12g/mol - 5=1.58 - ke=515L/molsec(at60C),Ea=26,400J/mol - ki2.55107Lmolsec( at 60C ). Fa 11,900J/mol - use activation energies to calculate ks and k at various temperatures. 2.4-Dichlorobenzoyl peroxide - M.W. =380g/mol - Assume f=0.5 - Obtain ks from nomograph Calculations: Fxamnla of numarinal matte ifor 6 non 1 whol. Graphical results: Methyl Methacrylate, 2,4-dichlorobenzoyl peroxide (a) 1phr Methyl Methacrylate, 2,4-dichlorobenzoyl peroxide, 50C Can you solve it in detail

solutions



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


