Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PPLLEEAASSEEEE SOMEONE SOLVE THIS!!!! Hint Ryan is a chemistry student that enjoys hot tea. He wants to determine how much ice is needed to cool
PPLLEEAASSEEEE SOMEONE SOLVE THIS!!!! 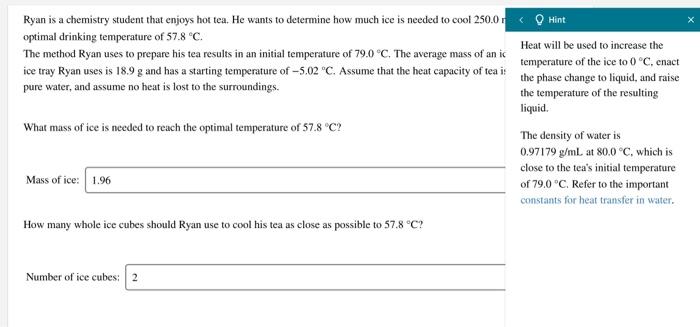
Hint Ryan is a chemistry student that enjoys hot tea. He wants to determine how much ice is needed to cool 250.0 optimal drinking temperature of 57.8 C. Heat will be used to increase the The method Ryan uses to prepare his tea results in an initial temperature of 79.0 C. The average mass of an ic ice tray Ryan uses is 18.9 g and has a starting temperature of -5.02 "C. Assume that the heat capacity of tea is the phase change to liquid, and raise temperature of the ice to 0C, cnact pure water, and assume no heat is lost to the surroundings. the temperature of the resulting liquid. What mass of ice is needed to reach the optimal temperature of 57.8C? The density of water is 0.97179 g/mL at 80.0C, which is close to the tea's initial temperature Mass of ice: 1.96 of 79,0 C. Refer to the important constants for heat transfer in water. How many whole ice cubes should Ryan use to cool his tea as close as possible to 57.8 C? Number of ice cubes: 2 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


