Question
Predict the bond order of Be2+ and Be. Do you expect these molecules to exist in the gas phase? Match the items in the
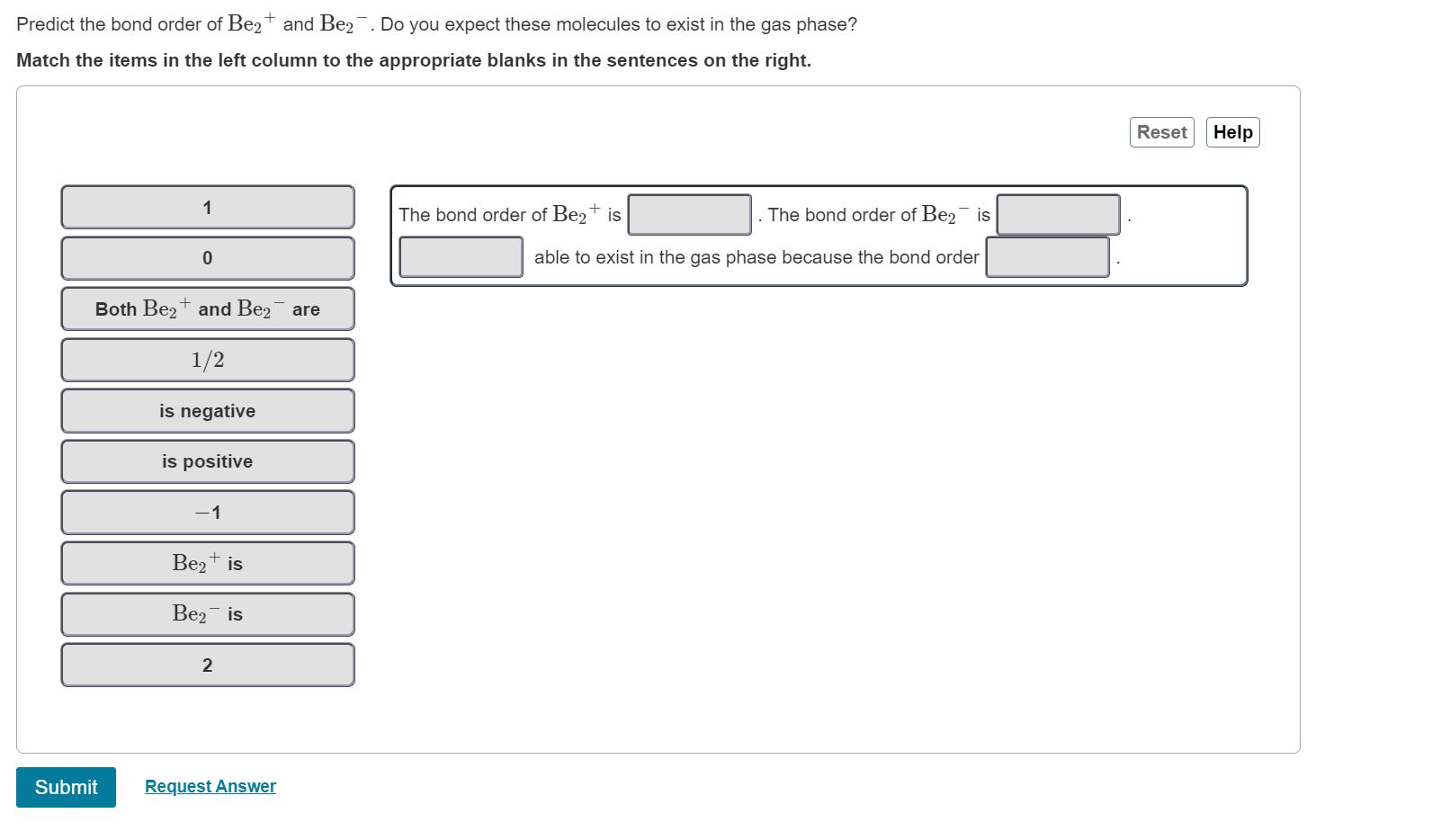
Predict the bond order of Be2+ and Be. Do you expect these molecules to exist in the gas phase? Match the items in the left column to the appropriate blanks in the sentences on the right. Su 1 0 Both Be+ and Be are 1/2 is negative is positive -1 Be is Be is 2 Request Answer The bond order of Be+ is The bond order of Be is able to exist in the gas phase because the bond order Reset Help
Step by Step Solution
3.54 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
by anti molecular orbital theory Be 2 has 7 electrons so its bond ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



