Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Pre-laboratory Assignment 4, Intravenous Sugar Solutions, Making Basic Solutions. Name Kristin O'Neill (i) - Normal saline solutions are used to clean wounds, help remove
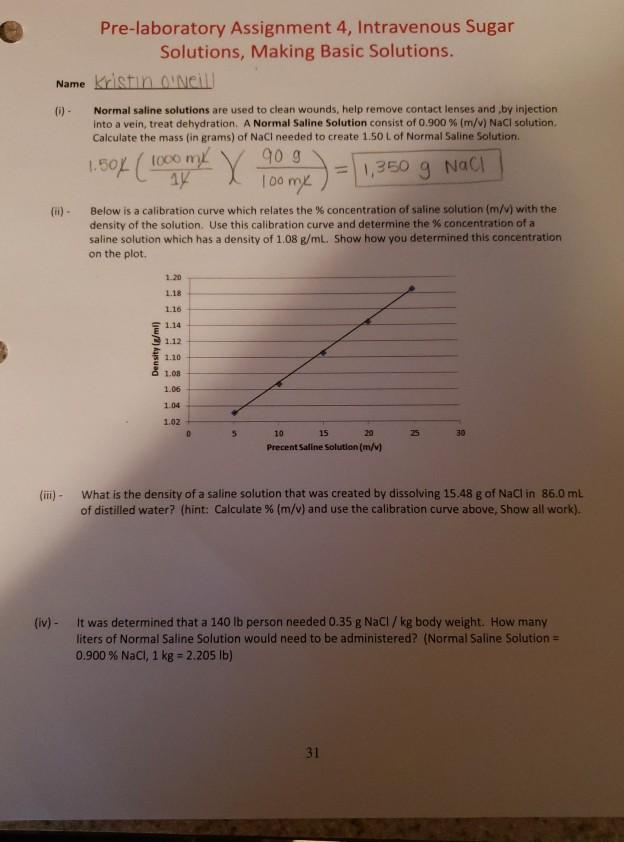
Pre-laboratory Assignment 4, Intravenous Sugar Solutions, Making Basic Solutions. Name Kristin O'Neill (i) - Normal saline solutions are used to clean wounds, help remove contact lenses and by injection into a vein, treat dehydration. A Normal Saline Solution consist of 0.900 % (m/v) NaCl solution. Calculate the mass (in grams) of NaCl needed to create 1.50 L of Normal Saline Solution. = 1,350 g NaCl 1.50% (1000 m X 90 g 100 mx Below is a calibration curve which relates the % concentration of saline solution (m/v) with the density of the solution. Use this calibration curve and determine the % concentration of a saline solution which has a density of 1.08 g/mL. Show how you determined this concentration on the plot. Density (g/ml) 1.20 1.18 1.16 1.14 1.12 1.10 1.08 1.06 1.04 1.02 5 10 15 20 Precent Saline Solution (m/v) 30 (iii) - What is the density of a saline solution that was created by dissolving 15.48 g of NaCl in 86.0 mL of distilled water? (hint: Calculate % (m/v) and use the calibration curve above, Show all work). (iv) - It was determined that a 140 lb person needed 0.35 g NaCl / kg body weight. How many liters of Normal Saline Solution would need to be administered? (Normal Saline Solution = 0.900 % NaCl, 1 kg = 2.205 lb) 31
Step by Step Solution
★★★★★
3.50 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
0900 mv Nall sol of Nall is present in Since In 100m...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


