Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Preparation of Solutions for Part B Preparation of Solutions for Part B Test Tube 1 Test Tube 2 Test Tube 3 Test Tube 4 Test
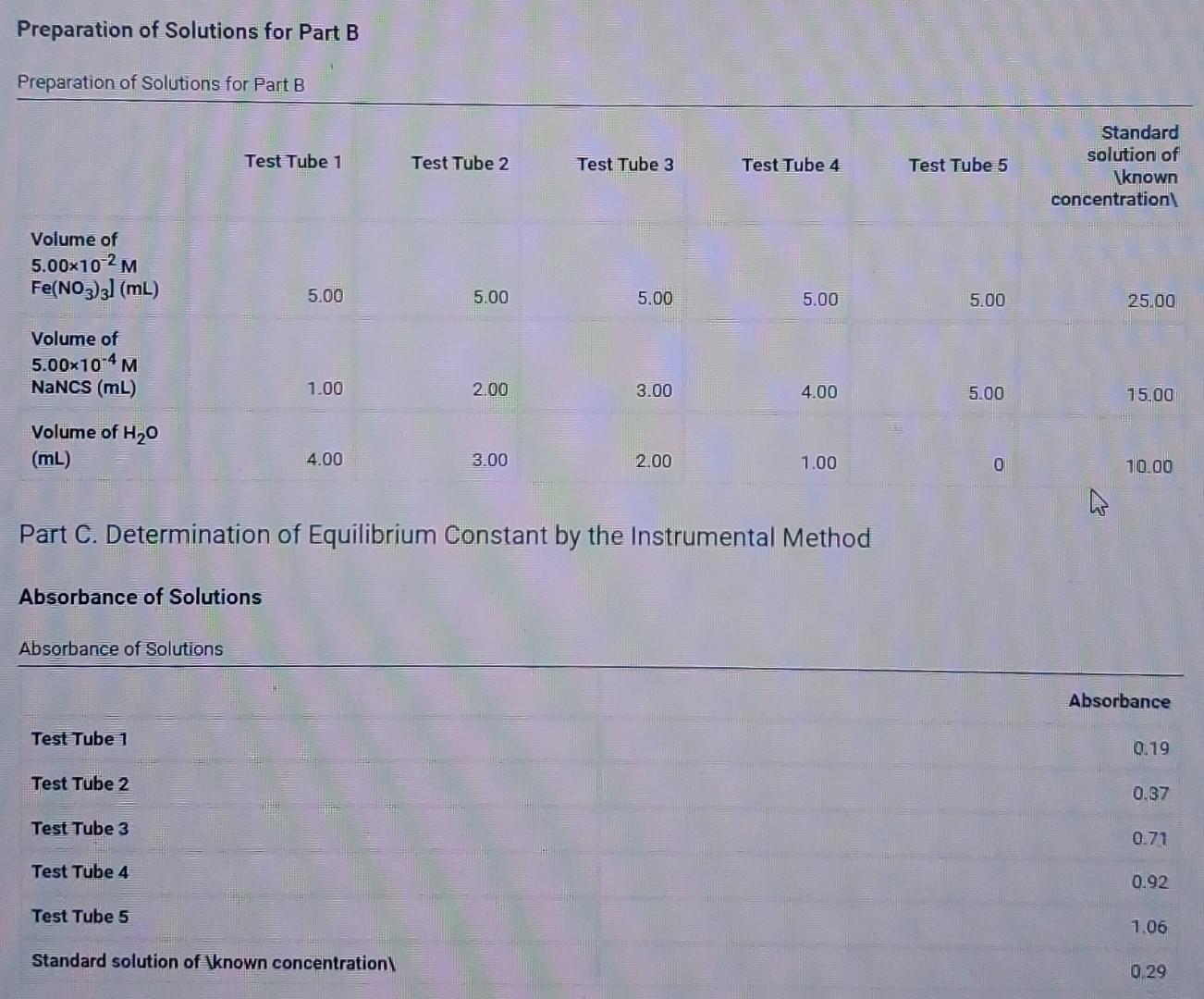
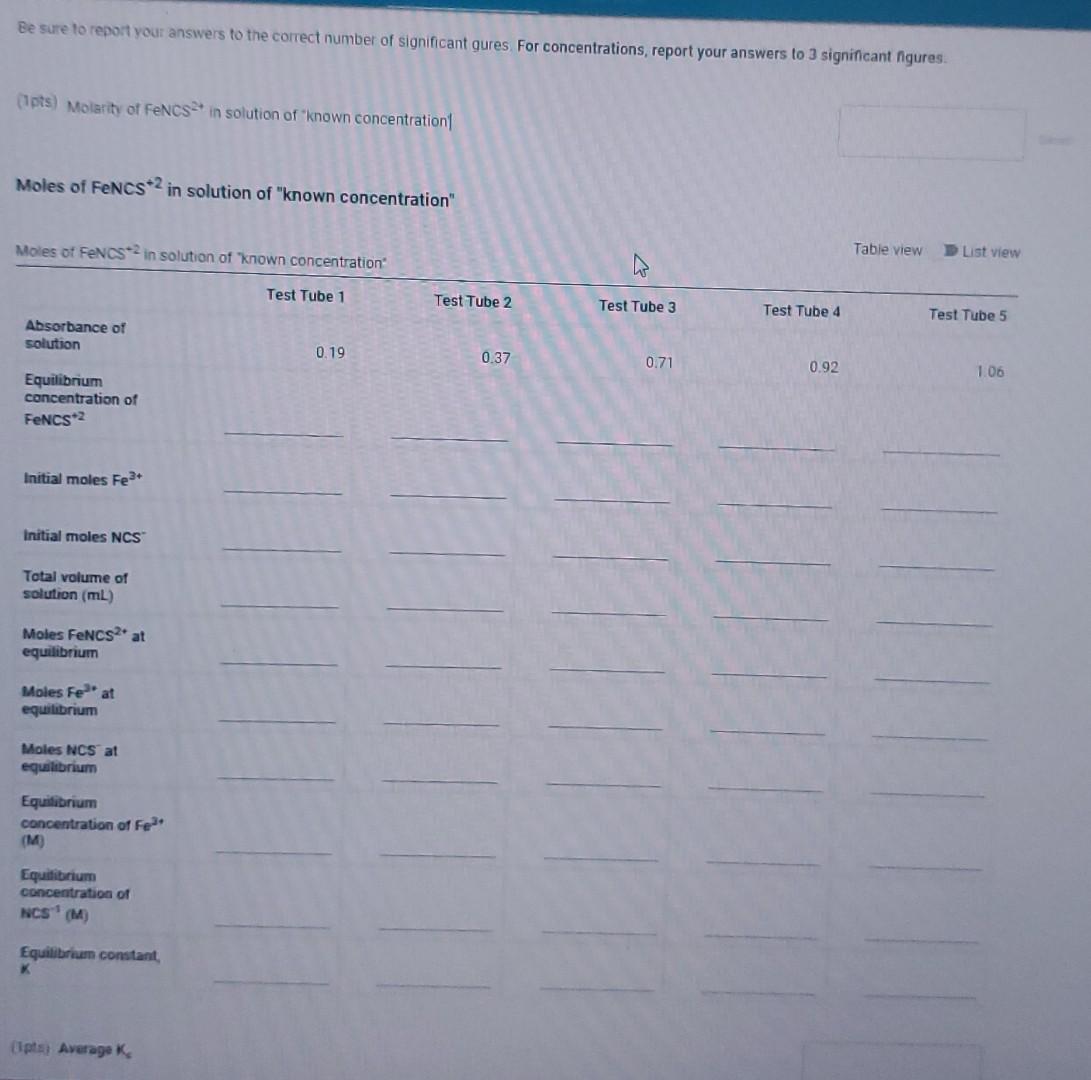
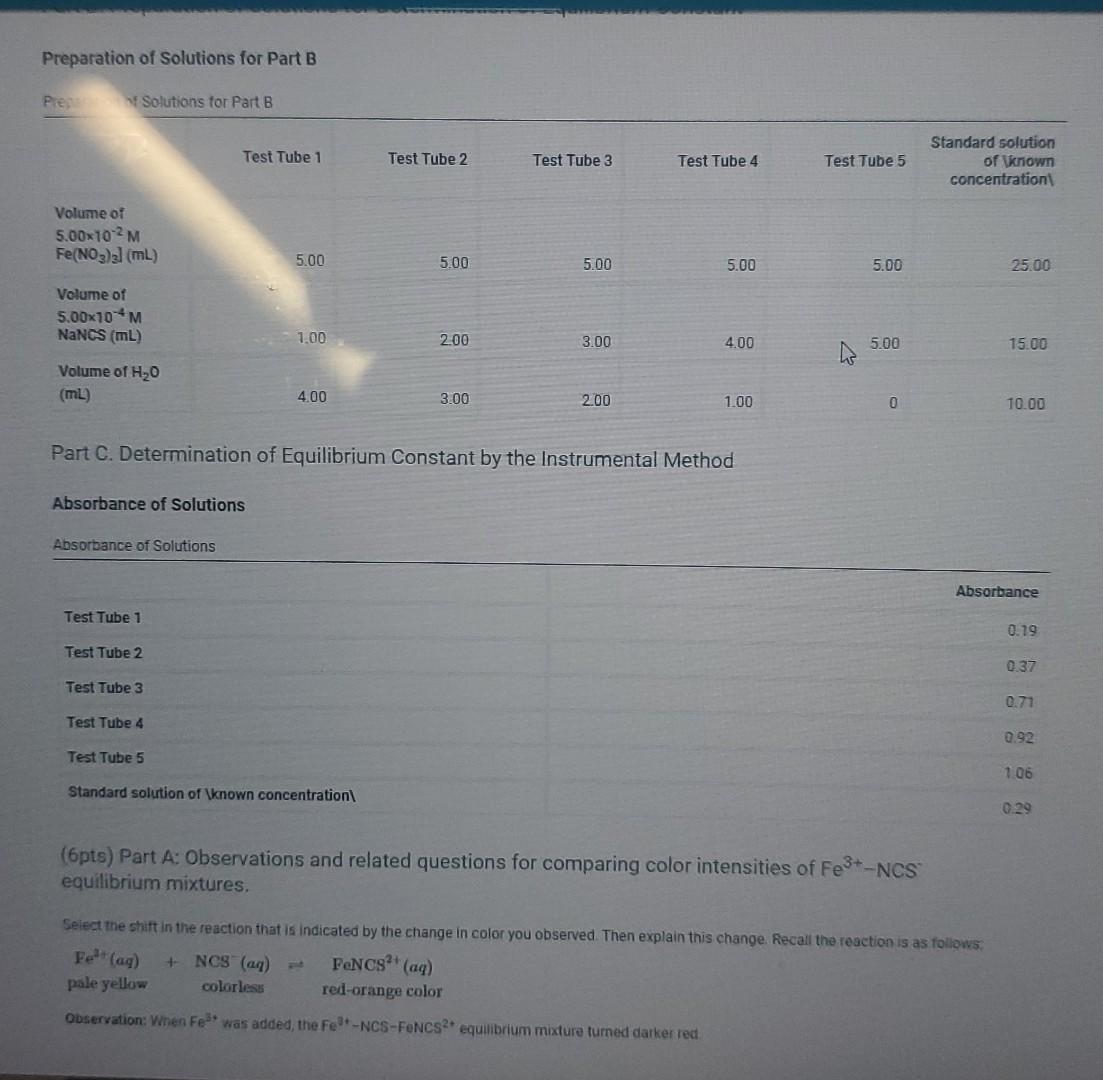
Preparation of Solutions for Part B Preparation of Solutions for Part B Test Tube 1 Test Tube 2 Test Tube 3 Test Tube 4 Test Tube 5 Standard solution of known concentration Volume of 5.00x102M Fe(NO3)3] (mL) 5.00 5.00 5.00 5.00 5.00 25.00 Volume of 5.00x10-4 M NaNCS (mL) 1.00 2.00 3.00 4.00 5.00 15.00 Volume of H20 (mL) 4.00 3.00 2.00 1.00 0 10.00 Part C. Determination of Equilibrium Constant by the Instrumental Method Absorbance of Solutions Absorbance of Solutions Absorbance Test Tube 1 0.19 Test Tube 2 0.37 Test Tube 3 0.71 Test Tube 4 0.92 Test Tube 5 1.06 Standard solution of \known concentration 0.29 Be sure to report your answers to the correct number of significant qures For concentrations, report your answers to 3 significant ngures. (Ipts) Molarity of FENCS in solution of known concentration Moles of FeNCS2 in solution of "known concentration" Moles of FENCS? in solution of "known concentration Table view List view W Test Tube 1 Test Tube 2 Test Tube 3 Test Tube 4 Test Tube 5 Absorbance of solution 0.19 0.37 0.71 0.92 1.06 Equilibrium concentration of FeNCS2 Initial moles Fe3+ Initial moles NCS Total volume of solution (m) Moles FeNCS? at equilibrium Moles Feat equilibrium Moles NCS at equilibrium Equilibrium concentration of Fe (M) Equilibrium concentration of NCS (M) Equilibrium constant lips Average Preparation of Solutions for Part B Pier Solutions for Part B Test Tube 1 Test Tube 2 Test Tube 3 Test Tube 4 Test Tube 5 Standard solution of known concentration Volume of 5.00x102M Fe(NO3)2 (mL) 5.00 5.00 5.00 5.00 5.00 25.00 Volume of 5.00x70 M NaNCS (ML) 1.00 200 3.00 4.00 5.00 15.00 Volume of H20 (ML) 4.00 3.00 2.00 1.00 0 10.00 Part C. Determination of Equilibrium Constant by the Instrumental Method Absorbance of Solutions Absorbance of Solutions Absorbance Test Tube 1 0.79 Test Tube 2 0.37 Test Tube 3 0.71 Test Tube 4 0.92 Test Tube 5 1.06 Standard solution of \known concentration 0.29 (6pts) Part A: Observations and related questions for comparing color intensities of Fe3+-NCS equilibrium mixtures, Select the shift in the reaction that is indicated by the change in color you observed. Then explain this change. Recall the reaction is as follows: Felt (ag) + NCS (a) PeNCS (aq) pale yellow colorless red-orange color Observation: When Fe was added, the Fe-NCS-FONCS equilibrium madure turned darker red
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


