Answered step by step
Verified Expert Solution
Question
1 Approved Answer
An adiabatic cylinder which contains an adiabatic frictionless piston Is connected to A steam pressure line. State 1: The volume under the piston is
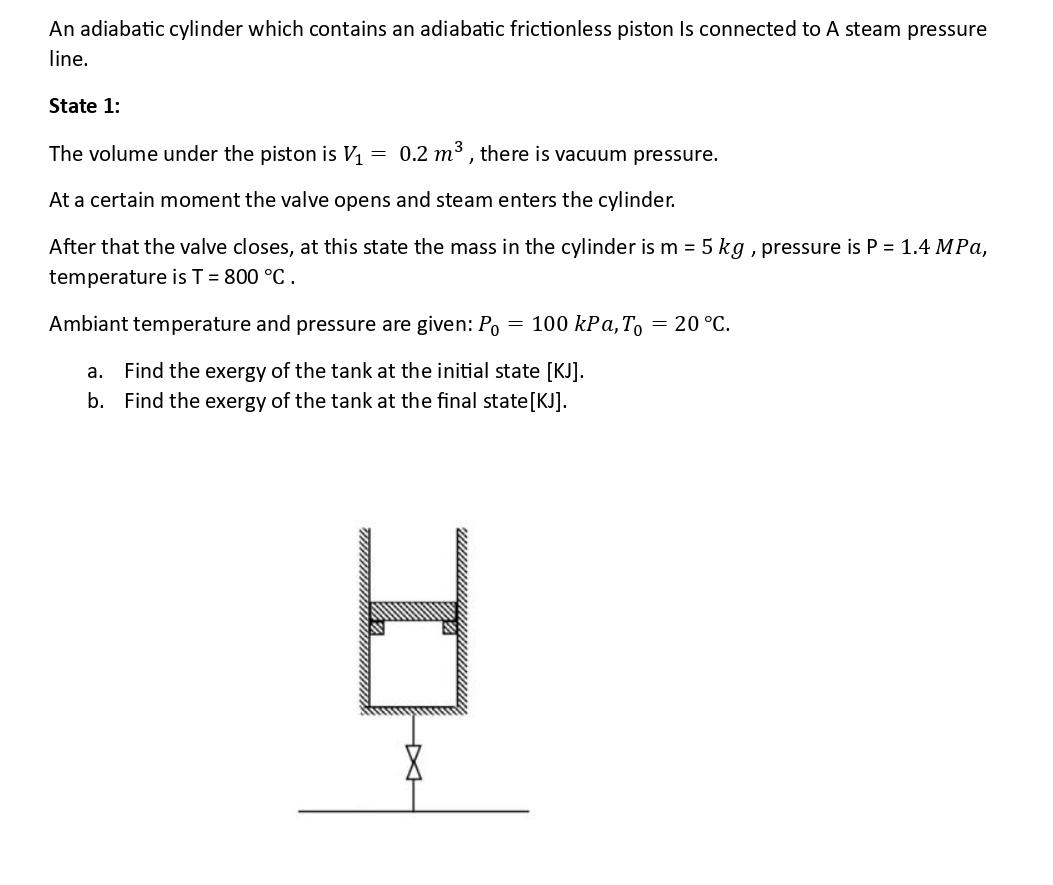
An adiabatic cylinder which contains an adiabatic frictionless piston Is connected to A steam pressure line. State 1: The volume under the piston is V = 0.2 m, there is vacuum pressure. At a certain moment the valve opens and steam enters the cylinder. After that the valve closes, at this state the mass in the cylinder is m = 5 kg, pressure is P = 1.4 MPa, temperature is T = 800 C. Ambiant temperature and pressure are given: Po = 100 kPa, To 20 C. a. Find the exergy of the tank at the initial state [KJ]. b. Find the exergy of the tank at the final state [KJ].
Step by Step Solution
★★★★★
3.63 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
SOLUTION a The exergy of the tank at the initial state is given by Exergy U PV T0S where U is the internal energy P is the pressure and V is the volume T0 is the ambient temperature and S is the entro...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


