Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the simple Linde-Hampson cycle shown in the figure. The gas is nitrogen and it is at 25C and 1 atm (101.325 kPa) at
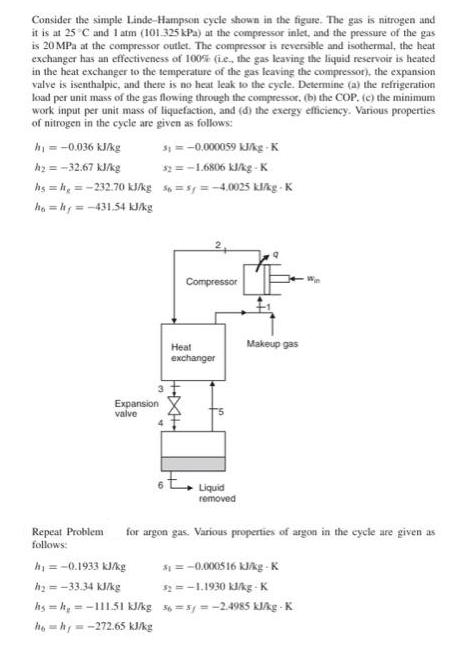
Consider the simple Linde-Hampson cycle shown in the figure. The gas is nitrogen and it is at 25C and 1 atm (101.325 kPa) at the compressor inlet, and the pressure of the gas is 20 MPa at the compressor outlet. The compressor is reversible and isothermal, the heat exchanger has an effectiveness of 100% (ie, the gas leaving the liquid reservoir is heated in the heat exchanger to the temperature of the gas leaving the compressor), the expansion valve is isenthalpic, and there is no heat leak to the cycle. Determine (a) the refrigeration load per unit mass of the gas flowing through the compressor, (b) the COP. (c) the minimum work input per unit mass of liquefaction, and (d) the exergy efficiency. Various properties of nitrogen in the cycle are given as follows: h = -0.036 kJ/kg h = -32.67 kJ/kg hs=h=-232.70 kJ/kg ho=hy=-431.54 kJ/kg Repeat Problem follows: Expansion valve 31 = -0,000059 kJ/kg-K 32 = -1.6806 kJ/kg-K h = -0.1933 kJ/kg h=-33.34 kJ/kg =s=-4.0025 kl/kg-K Compressor Heat exchanger Makeup gas Liquid removed for argon gas. Various properties of argon in the cycle are given as =-0.000516 kJ/kg-K $2=-1.1930 kJ/kg-K hs=h=-111.51 kJ/kg 36=5y=-2.4985 kJ/kg-K ho=hy=-272.65 kJ/kg
Step by Step Solution
★★★★★
3.56 Rating (170 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


