Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Prob. 1: Iron oxide is reduced to iron in an electric furnace in accordance with the following reaction: 4Fe2O3+9C=8Fe+6CO+3CO2 Required: (1) the number of kgs.
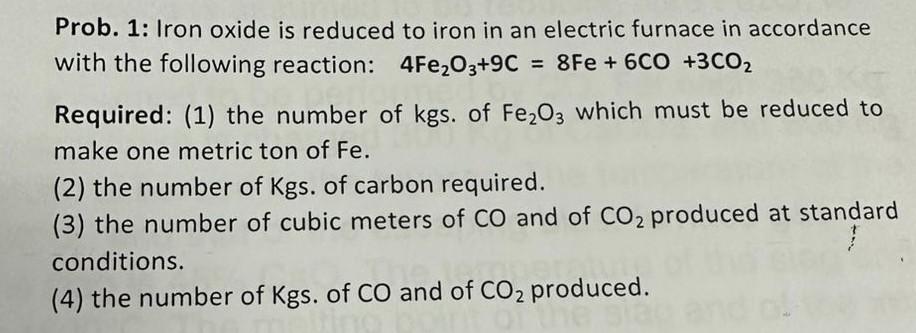
Prob. 1: Iron oxide is reduced to iron in an electric furnace in accordance with the following reaction: 4Fe2O3+9C=8Fe+6CO+3CO2 Required: (1) the number of kgs. of Fe2O3 which must be reduced to make one metric ton of Fe. (2) the number of Kgs. of carbon required. (3) the number of cubic meters of CO and of CO2 produced at standard conditions. (4) the number of Kgs. of CO and of CO2 produced
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


