Answered step by step
Verified Expert Solution
Question
1 Approved Answer
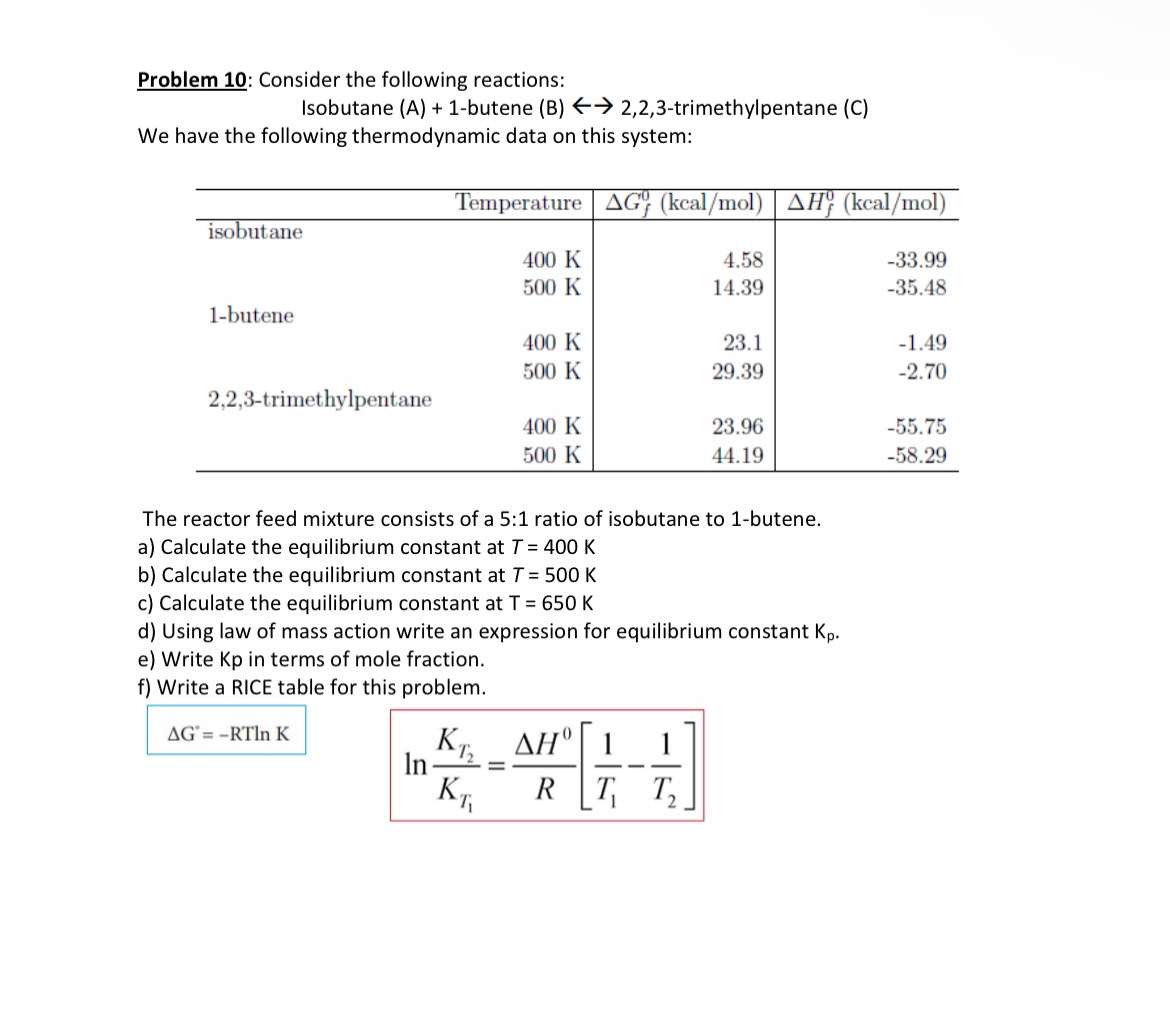
Problem 1 0 : Consider the following reactions: Isobutane ( A ) + 1 - butene ( B ) larr 2 , 2 , 3
Problem : Consider the following reactions:
Isobutane Abutene B larr trimethylpentane C
We have the following thermodynamic data on this system:
The reactor feed mixture consists of a : ratio of isobutane to butene.
a Calculate the equilibrium constant at
b Calculate the equilibrium constant at
c Calculate the equilibrium constant at
d Using law of mass action write an expression for equilibrium constant
e Write Kp in terms of mole fraction.
f Write a RICE table for this problem.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


