Answered step by step
Verified Expert Solution
Question
1 Approved Answer
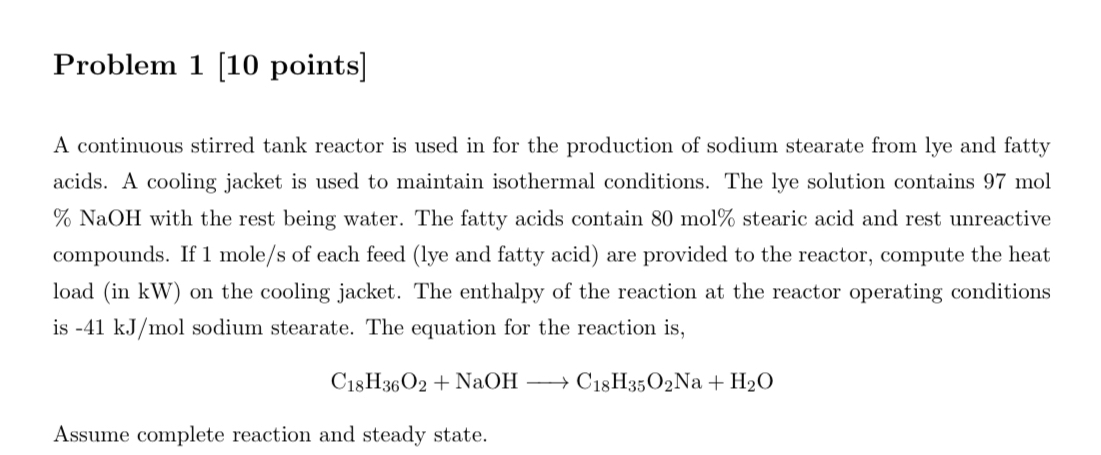
Problem 1 [ 1 0 points ] A continuous stirred tank reactor is used in for the production of sodium stearate from lye and fatty
Problem points
A continuous stirred tank reactor is used in for the production of sodium stearate from lye and fatty acids. A cooling jacket is used to maintain isothermal conditions. The lye solution contains mol NaOH with the rest being water. The fatty acids contain mol stearic acid and rest unreactive compounds. If mol of each feed lye and fatty acid are provided to the reactor, compute the heat load in on the cooling jacket. The enthalpy of the reaction at the reactor operating conditions is sodium stearate. The equation for the reaction is
Assume complete reaction and steady state.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


